Minimally Invasive Surgery in Pediatric Surgical Oncology
Abstract
1. Introduction
Fundamental Questions Surrounding MIS Resection of Pediatric Cancer
- How does the minimally invasive approach affect the ability to achieve a complete or gross total resection, negative margin status, and adequate lymph node sampling for a given tumor type?
- How does MIS impact relapse-free and overall survival?
- What patient and tumor characteristics should be considered during patient selection?
- What are the technical considerations?
2. History of MIS for Treating Pediatric Malignancy
3. Efficacy of MIS to Treat Pediatric Malignancy
3.1. Oncologic Integrity
3.2. Patient Selection
3.3. Benefits of MIS
4. Additional Applications of MIS
5. Limitations of MIS to Treat Pediatric Malignancy
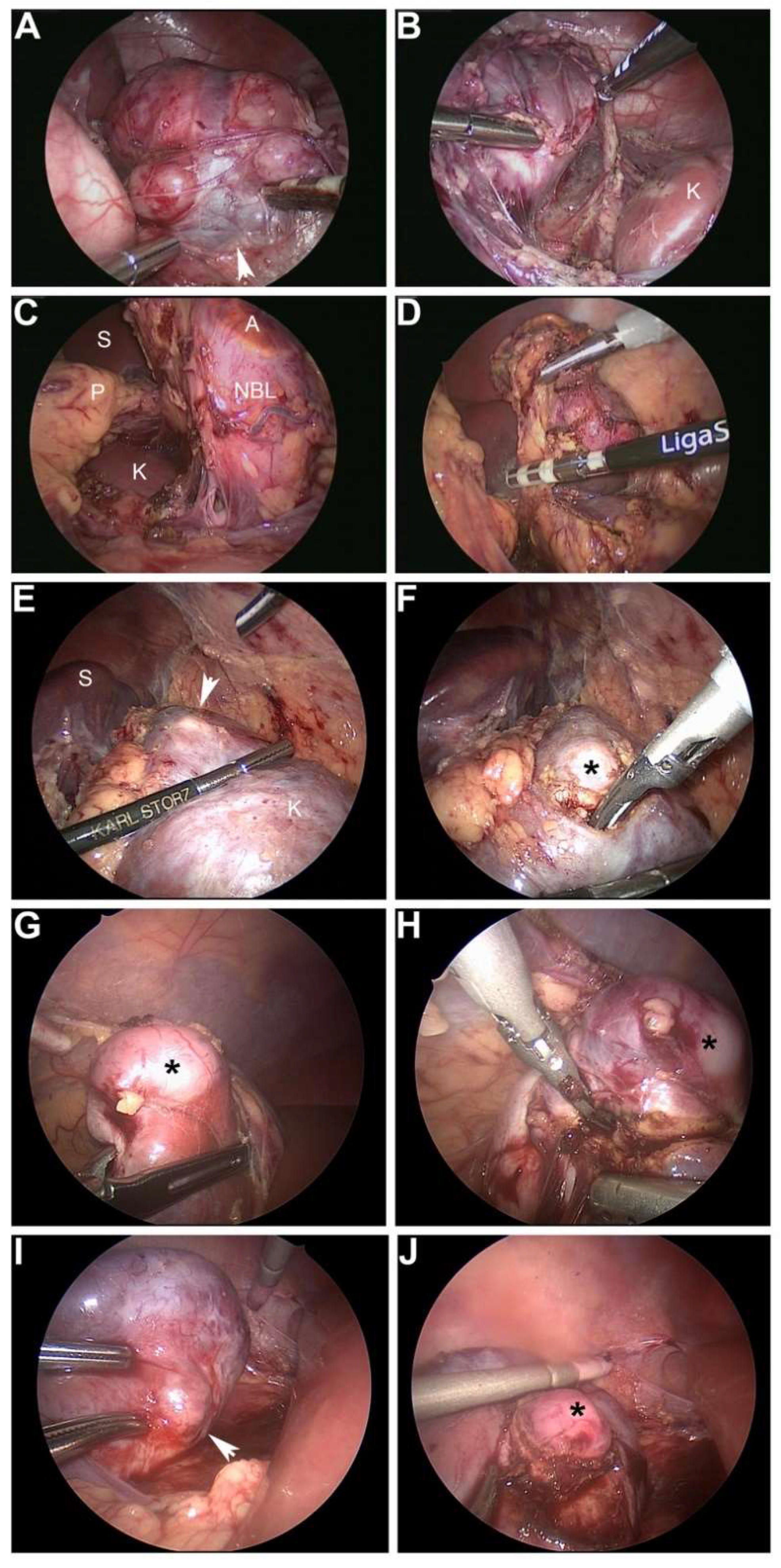
6. Technical Considerations: Pearls and Pitfalls of the MIS Approach
6.1. Technical Pearls
- Identify appropriate patient and cancer type.
- Leverage neoadjuvant chemotherapy to shrink tumors to facilitate resection when appropriate (e.g., no vasvular encasement and manageable tumor volume).
- Position patient for success.
- Commit to the challenge of completing procedure with MIS.
- Memorize preoperative imaging and location of vascular and other vital structures (and display images during procedure for frequent reference).
- Must achieve the appropriate oncologic principles for the specific tumor type.
- Complete or gross total (>98%) resection of neuroblastoma. *
- Complete resection of Wilms tumor without spill and adequate lymph node sampling.
- Have laparoscopic suction in field for short bursts to aspirate angiogenic bleeding, especially if resecting a tumor after neoadjuvant therapy.
- Recommend ultrasonic scalpel when excising mass from kidney or liver to preserve margin analysis given less thermal spread. Bipolar vessel sealers are adequate as well if greater distance from specimen is permissible and when dividing larger vessels.
- Surgical clips or vascular staplers are good for dividing larger vessels.
- Monitor progress regarding time, blood loss, and oncologic integrity. Be prepared to open if absolutely necessary.
- Use a specimen bag to remove the tumor.
- Identify a port location having good cosmesis to deliver specimen in a bag.
- Lengthen this ideal port to the narrowest diameter of tumor and deliver in that dimension.
6.2. Technical Pitfalls
- Inappropriate patient selection:
- Excessively large tumors.
- Vascular encasement.
- Limited tactile feedback, or haptics, with MIS instruments to appreciate large vessels, so must have good visualization of critical structures.
- Not mentally committing to MIS approach. These procedures are challenging.
- Not positioning patient appropriately.
- Tissue planes are difficult after neoadjuvant therapy (desmoplasia is challenging to dissect) and if angiogenic bleeding is excessive.
- Not recognizing feeding and draining vessels (i.e., difficult to control large anatomic vessels with MIS, so need to know location precisely).
- Not sampling lymph nodes adequately.
- Not completing the appropriate oncologic operation for a given tumor type.
- Recommend using a specimen bag.
- Do not morcellate tumor to deliver specimen—it must remain intact.
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Selby, L.V.; DeMatteo, R.P.; Tholey, R.M.; Jarnagin, W.R.; Garcia-Aguilar, J.; Strombom, P.D.; Allen, P.J.; Kingham, T.P.; Weiser, M.R.; Brennan, M.F.; et al. Evolving application of minimally invasive cancer operations at a tertiary cancer center. J. Surg. Oncol. 2017, 115, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Raoof, M.; Nota, C.; Melstrom, L.G.; Warner, S.G.; Woo, Y.; Singh, G.; Fong, Y. Oncologic outcomes after robot-assisted versus laparoscopic distal pancreatectomy: Analysis of the national cancer database. J. Surg. Oncol. 2018, 118, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Huscher, C.G.; Mingoli, A.; Sgarzini, G.; Sansonetti, A.; Di Paola, M.; Recher, A.; Ponzano, C. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: Five-year results of a randomized prospective trial. Ann. Surg. 2005, 241, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Aziz, O.; Constantinides, V.; Tekkis, P.P.; Athanasiou, T.; Purkayastha, S.; Paraskeva, P.; Darzi, A.W.; Heriot, A.G. Laparoscopic versus open surgery for rectal cancer: A meta-analysis. Ann. Surg. Oncol. 2006, 13, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Simillis, C.; Constantinides, V.A.; Tekkis, P.P.; Darzi, A.; Lovegrove, R.; Jiao, L.; Antoniou, A. Laparoscopic versus open hepatic resections for benign and malignant neoplasms—A meta-analysis. Surgery 2007, 141, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, G.W., 3rd. Minimally invasive surgery for solid tumors. Semin. Surg. Oncol. 1999, 16, 184–192. [Google Scholar] [CrossRef]
- Spurbeck, W.W.; Davidoff, A.M.; Lobe, T.E.; Rao, B.N.; Schropp, K.P.; Shochat, S.J. Minimally invasive surgery in pediatric cancer patients. Ann. Surg. Oncol. 2004, 11, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Javid, P.J.; Lendvay, T.S.; Acierno, S.; Gow, K.W. Laparoscopic nephroureterectomy for wilms’ tumor: Oncologic considerations. J. Pediatr. Surg. 2011, 46, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Metzelder, M.L.; Kuebler, J.F.; Shimotakahara, A.; Glueer, S.; Grigull, L.; Ure, B.M. Role of diagnostic and ablative minimally invasive surgery for pediatric malignancies. Cancer 2007, 109, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Malek, M.M.; Mollen, K.P.; Kane, T.D.; Shah, S.R.; Irwin, C. Thoracic neuroblastoma: A retrospective review of our institutional experience with comparison of the thoracoscopic and open approaches to resection. J. Pediatr. Surg. 2010, 45, 1622–1626. [Google Scholar] [CrossRef] [PubMed]
- Fraga, J.C.; Rothenberg, S.; Kiely, E.; Pierro, A. Video-assisted thoracic surgery resection for pediatric mediastinal neurogenic tumors. J. Pediatr. Surg. 2012, 47, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, C.M.; Smithson, L.; Nguyen, L.L.; Casadiego, G.; Nasr, A.; Irwin, M.S.; Gerstle, J.T. Clinical outcomes in children with adrenal neuroblastoma undergoing open versus laparoscopic adrenalectomy. J. Pediatr. Surg. 2013, 48, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Irtan, S.; Brisse, H.J.; Minard-Colin, V.; Schleiermacher, G.; Canale, S.; Sarnacki, S. Minimally invasive surgery of neuroblastic tumors in children: Indications depend on anatomical location and image-defined risk factors. Pediatr. Blood Cancer 2015, 62, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Phelps, H.M.; Ayers, G.D.; Ndolo, J.M.; Dietrich, H.L.; Watson, K.D.; Hilmes, M.A.; Lovvorn, H.N., 3rd. Maintaining oncologic integrity with minimally invasive resection of pediatric embryonal tumors. Surgery 2018, 164, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Rauth, T.P.; Slone, J.; Crane, G.; Correa, H.; Friedman, D.L.; Lovvorn, H.N., 3rd. Laparoscopic nephron-sparing resection of synchronous wilms tumors in a case of hyperplastic perilobar nephroblastomatosis. J. Pediatr. Surg. 2011, 46, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Warmann, S.W.; Godzinski, J.; van Tinteren, H.; Heij, H.; Powis, M.; Sandstedt, B.; Graf, N.; Fuchs, J. Surgical Panel of the SIOP Renal Tumor Strategy Group. Minimally invasive nephrectomy for wilms tumors in children–data from siop 2001. J. Pediatr. Surg. 2014, 49, 1544–1548. [Google Scholar] [CrossRef] [PubMed]
- Leclair, M.D.; de Lagausie, P.; Becmeur, F.; Varlet, F.; Thomas, C.; Valla, J.S.; Petit, T.; Philippe-Chomette, P.; Mure, P.Y.; Sarnacki, S.; et al. Laparoscopic resection of abdominal neuroblastoma. Ann. Surg. Oncol. 2008, 15, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Van Dalen, E.C.; de Lijster, M.S.; Leijssen, L.G.; Michiels, E.M.; Kremer, L.C.; Caron, H.N.; Aronson, D.C. Minimally invasive surgery versus open surgery for the treatment of solid abdominal and thoracic neoplasms in children. Cochrane Database Syst. Rev. 2015, 1, CD008403. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, P.F.; Newman, K.D.; Haase, G.M.; Lobe, T.E.; Wiener, E.S.; Holcomb, G.W. Lessons learned from a failed multi-institutional randomized controlled study. J. Pediatr. Surg. 2002, 37, 431–436. [Google Scholar] [CrossRef] [PubMed]
- American College of Surgeons. National cancer database. Available online: https://www.facs.org/quality-programs/cancer/ncdb (accessed on 18 July 2018).
- Ezekian, B.; Englum, B.R.; Gulack, B.C.; Rialon, K.L.; Kim, J.; Talbot, L.J.; Adibe, O.O.; Routh, J.C.; Tracy, E.T.; Rice, H.E. Comparing oncologic outcomes after minimally invasive and open surgery for pediatric neuroblastoma and wilms tumor. Pediatr. Blood Cancer 2018, 65. [Google Scholar] [CrossRef] [PubMed]
- Iwanaka, T.; Arai, M.; Ito, M.; Kawashima, H.; Yamamoto, K.; Hanada, R.; Imaizumi, S. Surgical treatment for abdominal neuroblastoma in the laparoscopic era. Surg. Endosc. 2001, 15, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Pineda, I.; Daw, N.C.; McCarville, B.; Emanus, L.J.; Rao, B.N.; Davidoff, A.M.; Shochat, S.J. Patients with osteosarcoma with a single pulmonary nodule on computed tomography: A single-institution experience. J. Pediatr. Surg. 2012, 47, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Pineda, I.; Seims, A.D.; VanHouwelingen, L.; Abdelhafeez, H.; Wu, H.; Wu, J.; Murphy, A.J.; Davidoff, A.M. Modified uniportal video-assisted thoracic surgery versus three-port approach for lung nodule biopsy in pediatric cancer patients. J. Laparoendosc. Adv. Surg. Tech. A 2018. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewski, J.J.; Sweeney, D.D.; Kavoussi, L.R.; Ost, M.C. Laparoscopic retroperitoneal lymph node dissection for high-risk pediatric patients with paratesticular rhabdomyosarcoma. J. Endourol. 2010, 24, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Rogers, E.M.; Casadiego Cubides, G.; Lacy, J.; Gerstle, J.T.; Kives, S.; Allen, L. Preoperative risk stratification of adnexal masses: Can we predict the optimal surgical management? J. Pediatr. Adolesc. Gynecol. 2014, 27, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Papic, J.C.; Finnell, S.M.; Slaven, J.E.; Billmire, D.F.; Rescorla, F.J.; Leys, C.M. Predictors of ovarian malignancy in children: Overcoming clinical barriers of ovarian preservation. J. Pediatr. Surg. 2014, 49, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Metzelder, M.; Kuebler, J.; Shimotakahara, A.; Vieten, G.; von Wasielewski, R.; Ure, B.M. Co(2) pneumoperitoneum increases systemic but not local tumor spread after intraperitoneal murine neuroblastoma spillage in mice. Surg. Endosc. 2008, 22, 2648–2653. [Google Scholar] [CrossRef] [PubMed]
- Reismann, M.; Wehrmann, F.; Schukfeh, N.; Kuebler, J.F.; Ure, B.; Gluer, S. Carbon dioxide, hypoxia and low ph lead to overexpression of c-myc and hmgb-1 oncogenes in neuroblastoma cells. Eur. J. Pediatr. Surg. 2009, 19, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, V.; Schaeff, B.; Schneider, M.; Gutt, C. Tumor seeding following laparoscopy: International survey. World J. Surg. 1999, 23, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Iwanaka, T.; Arai, M.; Yamamoto, H.; Fukuzawa, M.; Kubota, A.; Kouchi, K.; Nio, M.; Satomi, A.; Sasaki, F.; Yoneda, A.; et al. No incidence of port-site recurrence after endosurgical procedure for pediatric malignancies. Pediatr. Surg. Int. 2003, 19, 200–203. [Google Scholar] [PubMed]
- Davidoff, A.M. Neuroblastoma. Semin. Pediatr. Surg. 2012, 21, 2–14. [Google Scholar] [CrossRef] [PubMed]

| Study | Total Procedures | Intent | Conversions | Complications |
|---|---|---|---|---|
| Spurbeck 2004 [7] | 64 laparoscopy | 27 diagnosis/evaluation 7 resection 1 Hodgkin’s disease 1 CML 1 ALL 1 large-cell lymphoma 1 ganglioneuroma 1 pheochromocytoma 1 mesothelioma 30 treatment of complication | 4 | 2 liver hematoma 1 bowel injury |
| 49 thoracoscopy | 7 evaluation 40 biopsy/resection of pulmonary lesion 2 treatment of complication | 14 | 2 intraoperative desaturation 1 intraoperative bleeding | |
| Metzelder 2007 [9] | 65 laparoscopy | 41 biopsy/staging 24 resection 6 NBL 1 lymphoma 3 ovarian cancer 4 suspicious liver lesions 2 suspicious kidney lesions 8 suspicious lesions, other | 16 | 1 bowel injury 2 intraoperative bleeding |
| 25 thoracoscopy | 14 biopsy/staging 11 resection 3 NBL 1 lymphoma 1 lung metastasis 6 unknown | 5 | 1 intraoperative bleeding | |
| Leclair 2008 [17] | 45 laparoscopy | 45 resection 45 NBL | 4 | 1 bowel obstruction due to entrapment in trocar orifice 1 ischemia of kidney 1 wound abscess |
| Malek 2010 [10] | 11 thoracoscopy | 11 resection 11 NBL | 0 | 2 Horner syndrome 1 severe atelectasis |
| Fraga 2012 [11] | 17 thoracoscopy | 17 resection 17 NBL | 0 | 2 Horner syndrome |
| Kelleher 2013 [12] | 18 laparoscopy | 18 resection 18 NBL | 2 | None reported |
| Warmann 2014 [16] | 24 laparoscopy | 24 resection 24 WT | 0 | 1 splenic injury |
| Irtan 2015 [13] | 19 laparoscopy | 19 resection 19 NBL | 0 | 1 renal atrophy |
| 20 thoracoscopy | 2 biopsy 2 NBL 18 resection 18 NBL | 3 | 1 Horner syndrome 3 chylothorax | |
| Phelps 2018 [14] | 17 laparoscopy | 17 resection 13 NBL 3 WT 1 RMS | 0 | No acute complications |
| 8 thoracoscopy | 8 resection 8 NBL | 0 | No acute complications | |
| 1 cystoscopy | 1 resection 1 RMS | 0 | No acute complications |
| Citation | MIS Resections | Conversions | GTR | Negative Margins | Lymph Nodes | Median Follow-Up | Relapse and Survival |
|---|---|---|---|---|---|---|---|
| Spurbeck 2004 [7] | 7 | 0/7 | NR | NR | NR | NR | NR |
| Metzelder 2007 [9] | 35 | 14/35 (40%) | NR | NR | NR | 39 mo | NR |
| Leclair 2008 [17] | 45 | 4/45 (9%) | 43/45 (96%) | 37/45 (82%) | NR | 28 mo | OS: 84% ± 8.1 EFS: 77% ± 9.1 1 local + metastatic relapse 1 local relapse 1 metastatic relapse 1 progressive disease |
| Malek 2010 [10] | 11 | 0/11 | NR | 3/7 (43%) | NR | NR | EFS: 9.1% OS: 100% 1 local relapse |
| Fraga 2012 [11] | 17 | 0/17 | 17/17 (100%) | 17/17 (100%) | NR | 16 mo | OS & EFS: 100% |
| Kelleher 2013 [12] | 18 | 2/18 (11%) | NR | NR | NR | L/I risk: 42 mo H risk: 19 mo | L/I risk: 5-yr EFS and OS 100% H risk: numbers too small to calculate, 1 death |
| Warmann 2014 [16] | 24 | 0/24 | 24/24 (100%) | 21/24 (88%) | 15/24 (63%) sampled | 47 mo | EFS: 95.8% OS: 100% |
| Irtan 2015 [13] | 37 | 3/37 (8%) | 32/37 (86%) | NR | NR | 25 mo | 5-yr OS: 97.7% 5-yr EFS: 97.7% 1 metastatic relapse |
| Phelps 2018 [14] | 26 | 0/26 | 17/18 (94%) | 9/20 (45%) | 6/26 (23%) sampled | 58 mo | 5-yr RFS: 0.90 (CI, 0.66–0.97) 5-yr OS: 1.00 (CI, 1.00–1.00) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phelps, H.M.; Lovvorn, III, H.N. Minimally Invasive Surgery in Pediatric Surgical Oncology. Children 2018, 5, 158. https://doi.org/10.3390/children5120158
Phelps HM, Lovvorn, III HN. Minimally Invasive Surgery in Pediatric Surgical Oncology. Children. 2018; 5(12):158. https://doi.org/10.3390/children5120158
Chicago/Turabian StylePhelps, Hannah M., and Harold N. Lovvorn, III. 2018. "Minimally Invasive Surgery in Pediatric Surgical Oncology" Children 5, no. 12: 158. https://doi.org/10.3390/children5120158
APA StylePhelps, H. M., & Lovvorn, III, H. N. (2018). Minimally Invasive Surgery in Pediatric Surgical Oncology. Children, 5(12), 158. https://doi.org/10.3390/children5120158





