Current and Emerging Therapies in the Management of Hypoxic Ischemic Encephalopathy in Neonates
Abstract
:1. Introduction
2. Current Standard of Care
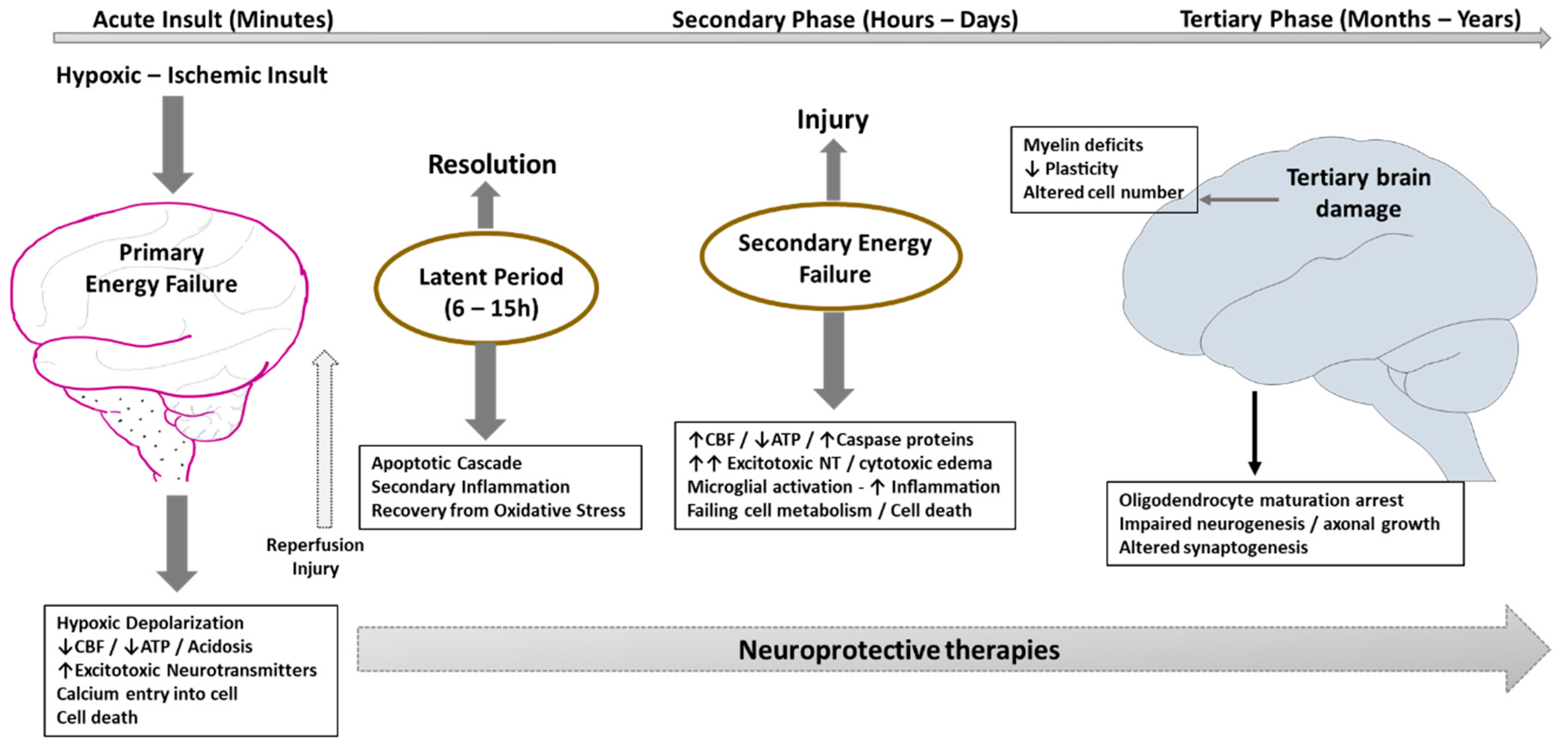
3. Pathophysiology of HIE and Implications for Intervention
4. Evaluation of HIE: Emerging Modalities
4.1. Biomarkers
4.1.1. Specific Biomarkers of Brain Injury
4.1.2. Inflammatory Markers
4.2. Metabolomic Analysis and Metabolites
4.3. Imaging
5. Emerging Therapies
5.1. Resuscitation
5.1.1. Optimizing Placental Transfusion
5.1.2. Vasopressin
5.2. Erythropietin/Analogues (Endogenous)
5.3. Stem Cells
5.4. Remote Ischemic Postconditioning (Endogenous)
5.5. Endocannabinoids (Endogenous)
5.6. Melatonin (Endogenous)
5.7. Monosialoganglioside
5.8. Xenon
5.9. Argon
5.10. Allopurinol
5.11. Magnesium Sulfate
5.12. Topiramate
5.13. Azithromycin
5.14. Combination Therapies
6. Management of Hypoxic Ischemic Injury beyond the Neonatal Period
7. Limitations of Management in Resource Poor Settings
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gaffey, M.F.; Das, J.K.; Bhutta, Z.A. Millennium development goals 4 and 5: Past and future progress. Semin. Fetal Neonatal. Med. 2015, 20, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, O.D. Reducing global neonatal mortality is possible. Neonatology 2011, 99, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Wyckoff, M.H.; Aziz, K.; Escobedo, M.B.; Kapadia, V.S.; Kattwinkel, J.; Perlman, J.M.; Simon, W.M.; Weiner, G.M.; Zaichkin, J.G. Part 13: Neonatal resuscitation: 2015 american heart association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015, 132 (Suppl. 2), S543–S560. [Google Scholar] [CrossRef] [PubMed]
- Shankaran, S.; Laptook, A.R.; Ehrenkranz, R.A.; Tyson, J.E.; McDonald, S.A.; Donovan, E.F.; Fanaroff, A.A.; Poole, W.K.; Wright, L.L.; Higgins, R.D.; et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med. 2005, 353, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Thoresen, M.; Tooley, J.; Liu, X.; Jary, S.; Fleming, P.; Luyt, K.; Jain, A.; Cairns, P.; Harding, D.; Sabir, H. Time is brain: Starting therapeutic hypothermia within three hours after birth improves motor outcome in asphyxiated newborns. Neonatology 2013, 104, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Committee on Fetus and Newborn; Papile, L.A.; Baley, J.E.; Benitz, W.; Cummings, J.; Carlo, W.A.; Eichenwald, E.; Kumar, P.; Polin, R.A.; Tan, R.C.; et al. Hypothermia and neonatal encephalopathy. Pediatrics 2014, 133, 1146–1150. [Google Scholar] [PubMed]
- Robertson, C.M.; Perlman, M. Follow-up of the term infant after hypoxic-ischemic encephalopathy. Paediatr. Child Health 2006, 11, 278–282. [Google Scholar] [PubMed]
- Fleiss, B.; Gressens, P. Tertiary mechanisms of brain damage: A new hope for treatment of cerebral palsy? Lancet Neurol. 2012, 11, 556–566. [Google Scholar] [CrossRef]
- Favrais, G.; van de Looij, Y.; Fleiss, B.; Ramanantsoa, N.; Bonnin, P.; Stoltenburg-Didinger, G.; Lacaud, A.; Saliba, E.; Dammann, O.; Gallego, J.; et al. Systemic inflammation disrupts the developmental program of white matter. Ann. Neurol. 2011, 70, 550–565. [Google Scholar] [CrossRef] [PubMed]
- Leviton, A.; Gressens, P. Neuronal damage accompanies perinatal white-matter damage. Trends Neurosci. 2007, 30, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Michetti, F.; Gazzolo, D. S100b protein in biological fluids: A tool for perinatal medicine. Clin. Chem. 2002, 48, 2097–2104. [Google Scholar] [PubMed]
- Zaigham, M.; Lundberg, F.; Olofsson, P. Protein s100b in umbilical cord blood as a potential biomarker of hypoxic-ischemic encephalopathy in asphyxiated newborns. Early Hum. Dev. 2017, 112, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Gazzolo, D.; Marinoni, E.; Di Iorio, R.; Bruschettini, M.; Kornacka, M.; Lituania, M.; Majewska, U.; Serra, G.; Michetti, F. Measurement of urinary s100b protein concentrations for the early identification of brain damage in asphyxiated full-term infants. Arch. Pediatr. Adolesc. Med. 2003, 157, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Massaro, A.N.; Wu, Y.W.; Bammler, T.K.; Comstock, B.; Mathur, A.; McKinstry, R.C.; Chang, T.; Mayock, D.E.; Mulkey, S.B.; Van Meurs, K.; et al. Plasma biomarkers of brain injury in neonatal hypoxic-ischemic encephalopathy. J. Pediatr. 2018, 194, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Celtik, C.; Acunas, B.; Oner, N.; Pala, O. Neuron-specific enolase as a marker of the severity and outcome of hypoxic ischemic encephalopathy. Brain Dev. 2004, 26, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Kelen, D.; Andorka, C.; Szabo, M.; Alafuzoff, A.; Kaila, K.; Summanen, M. Serum copeptin and neuron specific enolase are markers of neonatal distress and long-term neurodevelopmental outcome. PLoS ONE 2017, 12, e0184593. [Google Scholar] [CrossRef] [PubMed]
- Nagdyman, N.; Komen, W.; Ko, H.K.; Muller, C.; Obladen, M. Early biochemical indicators of hypoxic-ischemic encephalopathy after birth asphyxia. Pediatr. Res. 2001, 49, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Gazzolo, D.; Abella, R.; Marinoni, E.; Di Iorio, R.; Li Volti, G.; Galvano, F.; Pongiglione, G.; Frigiola, A.; Bertino, E.; Florio, P. Circulating biochemical markers of brain damage in infants complicated by ischemia reperfusion injury. Cardiovasc. Hematol. Agents Med. Chem. 2009, 7, 108–126. [Google Scholar] [CrossRef] [PubMed]
- Blennow, M.; Hagberg, H.; Rosengren, L. Glial fibrillary acidic protein in the cerebrospinal fluid: A possible indicator of prognosis in full-term asphyxiated newborn infants? Pediatr. Res. 1995, 37, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Chalak, L.F.; Sanchez, P.J.; Adams-Huet, B.; Laptook, A.R.; Heyne, R.J.; Rosenfeld, C.R. Biomarkers for severity of neonatal hypoxic-ischemic encephalopathy and outcomes in newborns receiving hypothermia therapy. J. Pediatr. 2014, 164, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Hasegawa, S.; Maeba, S.; Fukunaga, S.; Motoyama, M.; Hamano, H.; Ichiyama, T. Serum tau protein level serves as a predictive factor for neurological prognosis in neonatal asphyxia. Brain Dev. 2014, 36, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Walsh, B.H.; Boylan, G.B.; Livingstone, V.; Kenny, L.C.; Dempsey, E.M.; Murray, D.M. Cord blood proteins and multichannel-electroencephalography in hypoxic-ischemic encephalopathy. Pediatr. Crit. Care Med. 2013, 14, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Chun, P.T.; McPherson, R.J.; Marney, L.C.; Zangeneh, S.Z.; Parsons, B.A.; Shojaie, A.; Synovec, R.E.; Juul, S.E. Serial plasma metabolites following hypoxic-ischemic encephalopathy in a nonhuman primate model. Dev. Neurosci. 2015, 37, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Iwata, O.; Iwata, S.; Bainbridge, A.; De Vita, E.; Matsuishi, T.; Cady, E.B.; Robertson, N.J. Supra- and sub-baseline phosphocreatine recovery in developing brain after transient hypoxia-ischaemia: Relation to baseline energetics, insult severity and outcome. Brain 2008, 131, 2220–2226. [Google Scholar] [CrossRef] [PubMed]
- Cady, E.B.; Iwata, O.; Bainbridge, A.; Wyatt, J.S.; Robertson, N.J. Phosphorus magnetic resonance spectroscopy 2 h after perinatal cerebral hypoxia-ischemia prognosticates outcome in the newborn piglet. J. Neurochem. 2008, 107, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Robertson, N.J.; Cox, I.J.; Cowan, F.M.; Counsell, S.J.; Azzopardi, D.; Edwards, A.D. Cerebral intracellular lactic alkalosis persisting months after neonatal encephalopathy measured by magnetic resonance spectroscopy. Pediatr. Res. 1999, 46, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Katheria, A.C.; Lakshminrusimha, S.; Rabe, H.; McAdams, R.; Mercer, J.S. Placental transfusion: A review. J. Perinatol. 2017, 37, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Andersson, O.; Hellstrom-Westas, L.; Andersson, D.; Domellof, M. Effect of delayed versus early umbilical cord clamping on neonatal outcomes and iron status at 4 months: A randomised controlled trial. BMJ 2011, 343, d7157. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K. Nutrition and the developing brain: Nutrient priorities and measurement. Am. J. Clin. Nutr. 2007, 85, 614S–620S. [Google Scholar] [PubMed]
- Spoljaric, A.; Seja, P.; Spoljaric, I.; Virtanen, M.A.; Lindfors, J.; Uvarov, P.; Summanen, M.; Crow, A.K.; Hsueh, B.; Puskarjov, M.; et al. Vasopressin excites interneurons to suppress hippocampal network activity across a broad span of brain maturity at birth. Proc. Natl. Acad. Sci. USA 2017, 114, E10819–E10828. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Gonzalez, F.F. Erythropoietin: A novel therapy for hypoxic-ischaemic encephalopathy? Dev. Med. Child. Neurol. 2015, 57 (Suppl. 3), 34–39. [Google Scholar] [CrossRef] [PubMed]
- Sugawa, M.; Sakurai, Y.; Ishikawa-Ieda, Y.; Suzuki, H.; Asou, H. Effects of erythropoietin on glial cell development; oligodendrocyte maturation and astrocyte proliferation. Neurosci. Res. 2002, 44, 391–403. [Google Scholar] [CrossRef]
- Nagai, A.; Nakagawa, E.; Choi, H.B.; Hatori, K.; Kobayashi, S.; Kim, S.U. Erythropoietin and erythropoietin receptors in human cns neurons, astrocytes, microglia, and oligodendrocytes grown in culture. J. Neuropathol. Exp. Neurol. 2001, 60, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K.; Chong, Z.Z.; Hou, J.; Shang, Y.C. Erythropoietin and oxidative stress. Curr. Neurovasc. Res. 2008, 5, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Calvert, J.W.; Zhang, J.H. Neonatal hypoxia/ischemia is associated with decreased inflammatory mediators after erythropoietin administration. Stroke 2005, 36, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Juul, S.E.; Beyer, R.P.; Bammler, T.K.; McPherson, R.J.; Wilkerson, J.; Farin, F.M. Microarray analysis of high-dose recombinant erythropoietin treatment of unilateral brain injury in neonatal mouse hippocampus. Pediatr. Res. 2009, 65, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Yis, U.; Kurul, S.H.; Kumral, A.; Tugyan, K.; Cilaker, S.; Yilmaz, O.; Genc, S.; Genc, K. Effect of erythropoietin on oxygen-induced brain injury in the newborn rat. Neurosci. Lett. 2008, 448, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Dzietko, M.; Felderhoff-Mueser, U.; Sifringer, M.; Krutz, B.; Bittigau, P.; Thor, F.; Heumann, R.; Buhrer, C.; Ikonomidou, C.; Hansen, H.H. Erythropoietin protects the developing brain against n-methyl-d-aspartate receptor antagonist neurotoxicity. Neurobiol. Dis. 2004, 15, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Kang, W.; Xu, F.; Cheng, X.; Zhang, Z.; Jia, L.; Ji, L.; Guo, X.; Xiong, H.; Simbruner, G.; et al. Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics 2009, 124, e218–e226. [Google Scholar] [CrossRef] [PubMed]
- Elmahdy, H.; El-Mashad, A.R.; El-Bahrawy, H.; El-Gohary, T.; El-Barbary, A.; Aly, H. Human recombinant erythropoietin in asphyxia neonatorum: Pilot trial. Pediatrics 2010, 125, e1135–e1142. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Bauer, L.A.; Ballard, R.A.; Ferriero, D.M.; Glidden, D.V.; Mayock, D.E.; Chang, T.; Durand, D.J.; Song, D.; Bonifacio, S.L.; et al. Erythropoietin for neuroprotection in neonatal encephalopathy: Safety and pharmacokinetics. Pediatrics 2012, 130, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Mathur, A.M.; Chang, T.; McKinstry, R.C.; Mulkey, S.B.; Mayock, D.E.; Van Meurs, K.P.; Rogers, E.E.; Gonzalez, F.F.; Comstock, B.A.; et al. High-dose erythropoietin and hypothermia for hypoxic-ischemic encephalopathy: A phase ii trial. Pediatrics 2016, 137, e20160190. [Google Scholar] [CrossRef] [PubMed]
- Juul, S.E.; Comstock, B.A.; Heagerty, P.J.; Mayock, D.E.; Goodman, A.M.; Hauge, S.; Gonzalez, F.; Wu, Y.W. High-dose erythropoietin for asphyxia and encephalopathy (heal): A randomized controlled trial—Background, aims, and study protocol. Neonatology 2018, 113, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Baserga, M.C.; Beachy, J.C.; Roberts, J.K.; Ward, R.M.; DiGeronimo, R.J.; Walsh, W.F.; Ohls, R.K.; Anderson, J.; Mayock, D.E.; Juul, S.E.; et al. Darbepoetin administration to neonates undergoing cooling for encephalopathy: A safety and pharmacokinetic trial. Pediatr. Res. 2015, 78, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Mitsialis, S.A.; Kourembanas, S. Stem cell-based therapies for the newborn lung and brain: Possibilities and challenges. Semin. Perinatol. 2016, 40, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Nabetani, M.; Shintaku, H.; Hamazaki, T. Future perspectives of cell therapy for neonatal hypoxic-ischemic encephalopathy. Pediatr. Res. 2018, 83, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Archambault, J.; Moreira, A.; McDaniel, D.; Winter, L.; Sun, L.; Hornsby, P. Therapeutic potential of mesenchymal stromal cells for hypoxic ischemic encephalopathy: A systematic review and meta-analysis of preclinical studies. PLoS ONE 2017, 12, e0189895. [Google Scholar] [CrossRef] [PubMed]
- Jellema, R.K.; Wolfs, T.G.; Lima Passos, V.; Zwanenburg, A.; Ophelders, D.R.; Kuypers, E.; Hopman, A.H.; Dudink, J.; Steinbusch, H.W.; Andriessen, P.; et al. Mesenchymal stem cells induce t-cell tolerance and protect the preterm brain after global hypoxia-ischemia. PLoS ONE 2013, 8, e73031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotten, C.M.; Murtha, A.P.; Goldberg, R.N.; Grotegut, C.A.; Smith, P.B.; Goldstein, R.F.; Fisher, K.A.; Gustafson, K.E.; Waters-Pick, B.; Swamy, G.K.; et al. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J. Pediatr. 2014, 164, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G.; Botker, H.E.; Przyklenk, K.; Redington, A.; Yellon, D. Remote ischemic conditioning. J. Am. Coll. Cardiol. 2015, 65, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Baillieul, S.; Chacaroun, S.; Doutreleau, S.; Detante, O.; Pepin, J.L.; Verges, S. Hypoxic conditioning and the central nervous system: A new therapeutic opportunity for brain and spinal cord injuries? Exp. Biol. Med. 2017, 242, 1198–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.Y.; Hausenloy, D.J. Remote ischemic conditioning: From bench to bedside. Front. Physiol. 2012, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Drunalini Perera, P.N.; Hu, Q.; Tang, J.; Li, L.; Barnhart, M.; Doycheva, D.M.; Zhang, J.H.; Tang, J. Delayed remote ischemic postconditioning improves long term sensory motor deficits in a neonatal hypoxic ischemic rat model. PLoS ONE 2014, 9, e90258. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Ferreira, E.; Rudge, B.; Hughes, M.P.; Rahim, A.A.; Hristova, M.; Robertson, N.J. Immediate remote ischemic postconditioning reduces brain nitrotyrosine formation in a piglet asphyxia model. Oxid. Med. Cell. Longev. 2016, 2016, 5763743. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lopez, D.; Martinez-Orgado, J.; Nunez, E.; Romero, J.; Lorenzo, P.; Moro, M.A.; Lizasoain, I. Characterization of the neuroprotective effect of the cannabinoid agonist win-55212 in an in vitro model of hypoxic-ischemic brain damage in newborn rats. Pediatr. Res. 2006, 60, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lopez, D.; Pradillo, J.M.; Garcia-Yebenes, I.; Martinez-Orgado, J.A.; Moro, M.A.; Lizasoain, I. The cannabinoid win55212-2 promotes neural repair after neonatal hypoxia-ischemia. Stroke 2010, 41, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.J.; Lafuente, H.; Rey-Santano, M.C.; Mielgo, V.E.; Gastiasoro, E.; Rueda, M.; Pertwee, R.G.; Castillo, A.I.; Romero, J.; Martinez-Orgado, J. Neuroprotective effects of the nonpsychoactive cannabinoid cannabidiol in hypoxic-ischemic newborn piglets. Pediatr. Res. 2008, 64, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Villapol, S.; Fau, S.; Renolleau, S.; Biran, V.; Charriaut-Marlangue, C.; Baud, O. Melatonin promotes myelination by decreasing white matter inflammation after neonatal stroke. Pediatr. Res. 2011, 69, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Jou, M.J.; Peng, T.I.; Yu, P.Z.; Jou, S.B.; Reiter, R.J.; Chen, J.Y.; Wu, H.Y.; Chen, C.C.; Hsu, L.F. Melatonin protects against common deletion of mitochondrial DNA-augmented mitochondrial oxidative stress and apoptosis. J. Pineal Res. 2007, 43, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; BaHammam, A.S.; Brown, G.M.; Spence, D.W.; Bharti, V.K.; Kaur, C.; Hardeland, R.; Cardinali, D.P. Melatonin antioxidative defense: Therapeutical implications for aging and neurodegenerative processes. Neurotox. Res. 2013, 23, 267–300. [Google Scholar] [CrossRef] [PubMed]
- Robertson, N.J.; Faulkner, S.; Fleiss, B.; Bainbridge, A.; Andorka, C.; Price, D.; Powell, E.; Lecky-Thompson, L.; Thei, L.; Chandrasekaran, M.; et al. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain 2013, 136, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.; Elmahdy, H.; El-Dib, M.; Rowisha, M.; Awny, M.; El-Gohary, T.; Elbatch, M.; Hamisa, M.; El-Mashad, A.R. Melatonin use for neuroprotection in perinatal asphyxia: A randomized controlled pilot study. J. Perinatol. 2015, 35, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Seabra, M.L.; Bignotto, M.; Pinto, L.R., Jr.; Tufik, S. Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J. Pineal Res. 2000, 29, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Fulia, F.; Gitto, E.; Cuzzocrea, S.; Reiter, R.J.; Dugo, L.; Gitto, P.; Barberi, S.; Cordaro, S.; Barberi, I. Increased levels of malondialdehyde and nitrite/nitrate in the blood of asphyxiated newborns: Reduction by melatonin. J. Pineal Res. 2001, 31, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Hendaus, M.A.; Jomha, F.A.; Alhammadi, A.H. Melatonin in the management of perinatal hypoxic-ischemic encephalopathy: Light at the end of the tunnel? Neuropsychiatr. Dis. Treat. 2016, 12, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.R.; Muraro, F.; Zylbersztejn, D.S.; Abel, C.R.; Arteni, N.S.; Lavinsky, D.; Netto, C.A.; Trindade, V.M. Neonatal hypoxia-ischemia reduces ganglioside, phospholipid and cholesterol contents in the rat hippocampus. Neurosci. Res. 2003, 46, 339–347. [Google Scholar] [CrossRef]
- Ferrari, G.; Anderson, B.L.; Stephens, R.M.; Kaplan, D.R.; Greene, L.A. Prevention of apoptotic neuronal death by gm1 ganglioside. Involvement of trk neurotrophin receptors. J. Biol. Chem. 1995, 270, 3074–3080. [Google Scholar] [CrossRef] [PubMed]
- Ballough, G.P.; Cann, F.J.; Smith, C.D.; Forster, J.S.; Kling, C.E.; Filbert, M.G. GM1 monosialoganglioside pretreatment protects against soman-induced seizure-related brain damage. Mol. Chem. Neuropathol. 1998, 34, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Li, Z. Adjuvant treatment with monosialoganglioside may improve neurological outcomes in neonatal hypoxic-ischemic encephalopathy: A meta-analysis of randomized controlled trials. PLoS ONE 2017, 12, e0183490. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, S.; Bainbridge, A.; Kato, T.; Chandrasekaran, M.; Kapetanakis, A.B.; Hristova, M.; Liu, M.; Evans, S.; De Vita, E.; Kelen, D.; et al. Xenon augmented hypothermia reduces early lactate/N-acetylaspartate and cell death in perinatal asphyxia. Ann. Neurol. 2011, 70, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Chakkarapani, E.; Dingley, J.; Liu, X.; Hoque, N.; Aquilina, K.; Porter, H.; Thoresen, M. Xenon enhances hypothermic neuroprotection in asphyxiated newborn pigs. Ann. Neurol. 2010, 68, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Azzopardi, D.; Robertson, N.J.; Bainbridge, A.; Cady, E.; Charles-Edwards, G.; Deierl, A.; Fagiolo, G.; Franks, N.P.; Griffiths, J.; Hajnal, J.; et al. Moderate hypothermia within 6 h of birth plus inhaled xenon versus moderate hypothermia alone after birth asphyxia (TOBY-Xe): A proof-of-concept, open-label, randomised controlled trial. Lancet Neurol. 2016, 15, 145–153. [Google Scholar] [CrossRef]
- Faulkner, S.D.; Downie, N.A.; Mercer, C.J.; Kerr, S.A.; Sanders, R.D.; Robertson, N.J. A xenon recirculating ventilator for the newborn piglet: Developing clinical applications of xenon for neonates. Eur. J. Anaesthesiol. 2012, 29, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Broad, K.D.; Fierens, I.; Fleiss, B.; Rocha-Ferreira, E.; Ezzati, M.; Hassell, J.; Alonso-Alconada, D.; Bainbridge, A.; Kawano, G.; Ma, D.; et al. Inhaled 45–50% argon augments hypothermic brain protection in a piglet model of perinatal asphyxia. Neurobiol. Dis. 2016, 87, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, L.; Yang, T.; Zhao, H.; Fidalgo, A.R.; Vizcaychipi, M.P.; Sanders, R.D.; Yu, B.; Takata, M.; Johnson, M.R.; Ma, D. The protective profile of argon, helium, and xenon in a model of neonatal asphyxia in rats. Crit. Care Med. 2012, 40, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Okamoto, K.; Kusano, T.; Matsuda, Y.; Suzuki, G.; Fuse, A.; Yokota, H. The effects of xanthine oxidoreductase inhibitors on oxidative stress markers following global brain ischemia reperfusion injury in C57BL/6 mice. PLoS ONE 2015, 10, e0133980. [Google Scholar] [CrossRef] [PubMed]
- Marro, P.J.; Mishra, O.P.; Delivoria-Papadopoulos, M. Effect of allopurinol on brain adenosine levels during hypoxia in newborn piglets. Brain Res. 2006, 1073–1074, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Fanjul, J.; Duran Fernandez-Feijoo, C.; Lopez-Abad, M.; Lopez Ramos, M.G.; Balada Caballe, R.; Alcantara-Horillo, S.; Camprubi Camprubi, M. Neuroprotection with hypothermia and allopurinol in an animal model of hypoxic-ischemic injury: Is it a gender question? PLoS ONE 2017, 12, e0184643. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, T.; McGuire, W. Allopurinol for preventing mortality and morbidity in newborn infants with hypoxic-ischaemic encephalopathy. Cochrane Database Syst. Rev. 2012, CD006817. [Google Scholar] [CrossRef] [PubMed]
- Kaandorp, J.J.; van Bel, F.; Veen, S.; Derks, J.B.; Groenendaal, F.; Rijken, M.; Roze, E.; Venema, M.M.; Rademaker, C.M.; Bos, A.F.; et al. Long-term neuroprotective effects of allopurinol after moderate perinatal asphyxia: Follow-up of two randomised controlled trials. Arch. Dis. Child. Fetal Neonatal. Ed. 2012, 97, F162–F166. [Google Scholar] [CrossRef] [PubMed]
- Torrance, H.L.; Benders, M.J.; Derks, J.B.; Rademaker, C.M.; Bos, A.F.; Van Den Berg, P.; Longini, M.; Buonocore, G.; Venegas, M.; Baquero, H.; et al. Maternal allopurinol during fetal hypoxia lowers cord blood levels of the brain injury marker s-100b. Pediatrics 2009, 124, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Ilves, P.; Kiisk, M.; Soopold, T.; Talvik, T. Serum total magnesium and ionized calcium concentrations in asphyxiated term newborn infants with hypoxic-ischaemic encephalopathy. Acta Paediatr. 2000, 89, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.A.; Charoo, B.A.; Bhat, J.I.; Ahmad, S.M.; Ali, S.W.; Mufti, M.U. Magnesium sulfate in severe perinatal asphyxia: A randomized, placebo-controlled trial. Pediatrics 2009, 123, e764–e769. [Google Scholar] [CrossRef] [PubMed]
- Galinsky, R.; Bennet, L.; Groenendaal, F.; Lear, C.A.; Tan, S.; van Bel, F.; Juul, S.E.; Robertson, N.J.; Mallard, C.; Gunn, A.J. Magnesium is not consistently neuroprotective for perinatal hypoxia-ischemia in term-equivalent models in preclinical studies: A systematic review. Dev. Neurosci. 2014, 36, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Lingam, I.; Robertson, N.J. Magnesium as a neuroprotective agent: A review of its use in the fetus, term infant with neonatal encephalopathy, and the adult stroke patient. Dev. Neurosci. 2018, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tagin, M.; Shah, P.S.; Lee, K.S. Magnesium for newborns with hypoxic-ischemic encephalopathy: A systematic review and meta-analysis. J. Perinatol. 2013, 33, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Tagin, M.; Abdel-Hady, H.; ur Rahman, S.; Azzopardi, D.V.; Gunn, A.J. Neuroprotection for perinatal hypoxic ischemic encephalopathy in low- and middle-income countries. J. Pediatr. 2015, 167, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Poggi, C.; la Marca, G.; Furlanetto, S.; Fiorini, P.; Cavallaro, G.; Plantulli, A.; Donzelli, G.; Guerrini, R. Oral topiramate in neonates with hypoxic ischemic encephalopathy treated with hypothermia: A safety study. J. Pediatr. 2010, 157, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Fiorini, P.; Catarzi, S.; Berti, E.; Padrini, L.; Landucci, E.; Donzelli, G.; Bartalena, L.; Fiorentini, E.; Boldrini, A.; et al. Safety and efficacy of topiramate in neonates with hypoxic ischemic encephalopathy treated with hypothermia (NeoNATI): A feasibility study. J. Matern. Fetal Neonatal. Med. 2018, 31, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Amantea, D.; Certo, M.; Petrelli, F.; Tassorelli, C.; Micieli, G.; Corasaniti, M.T.; Puccetti, P.; Fallarino, F.; Bagetta, G. Azithromycin protects mice against ischemic stroke injury by promoting macrophage transition towards m2 phenotype. Exp. Neurol. 2016, 275 Pt 1, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Barks, J.L.Y.; Silverstein, F. Repurposing Azithromycin for Neonatal Neuroprotection: Next Steps; PAS: Toronto, ON, Canada, 2018. [Google Scholar]
- Murray, D.M.; O’Connor, C.M.; Ryan, C.A.; Korotchikova, I.; Boylan, G.B. Early EEG grade and outcome at 5 years after mild neonatal hypoxic ischemic encephalopathy. Pediatrics 2016, 138, e20160659. [Google Scholar] [CrossRef] [PubMed]
- Robertson, N.J.; Tan, S.; Groenendaal, F.; van Bel, F.; Juul, S.E.; Bennet, L.; Derrick, M.; Back, S.A.; Valdez, R.C.; Northington, F.; et al. Which neuroprotective agents are ready for bench to bedside translation in the newborn infant? J. Pediatr. 2012, 160, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Covey, M.V.; Loporchio, D.; Buono, K.D.; Levison, S.W. Opposite effect of inflammation on subventricular zone versus hippocampal precursors in brain injury. Ann. Neurol. 2011, 70, 616–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fancy, S.P.; Harrington, E.P.; Yuen, T.J.; Silbereis, J.C.; Zhao, C.; Baranzini, S.E.; Bruce, C.C.; Otero, J.J.; Huang, E.J.; Nusse, R.; et al. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat. Neurosci. 2011, 14, 1009–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buser, J.R.; Maire, J.; Riddle, A.; Gong, X.; Nguyen, T.; Nelson, K.; Luo, N.L.; Ren, J.; Struve, J.; Sherman, L.S.; et al. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann. Neurol. 2012, 71, 93–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, I.C.; Champagne, F.A.; Brown, S.E.; Dymov, S.; Sharma, S.; Meaney, M.J.; Szyf, M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: Altering epigenetic marking later in life. J. Neurosci. 2005, 25, 11045–11054. [Google Scholar] [CrossRef] [PubMed]
- Faraco, G.; Pancani, T.; Formentini, L.; Mascagni, P.; Fossati, G.; Leoni, F.; Moroni, F.; Chiarugi, A. Pharmacological inhibition of histone deacetylases by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain. Mol. Pharmacol. 2006, 70, 1876–1884. [Google Scholar] [CrossRef] [PubMed]
- Brogdon, J.L.; Xu, Y.; Szabo, S.J.; An, S.; Buxton, F.; Cohen, D.; Huang, Q. Histone deacetylase activities are required for innate immune cell control of th1 but not Th2 effector cell function. Blood 2007, 109, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.D.; Banerjee, R.; Liu, J.; Gendelman, H.E.; Mosley, R.L. Neuroprotective activities of CD4+CD25+ regulatory t cells in an animal model of parkinson’s disease. J. Leukoc. Biol. 2007, 82, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, E.D.; Veremeyko, T.; Barteneva, N.; Krichevsky, A.M.; Weiner, H.L. Microrna-124 promotes microglia quiescence and suppresses eae by deactivating macrophages via the c/ebp-alpha-pu.1 pathway. Nat. Med. 2011, 17, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Covey, M.V.; Jiang, Y.; Alli, V.V.; Yang, Z.; Levison, S.W. Defining the critical period for neocortical neurogenesis after pediatric brain injury. Dev. Neurosci. 2010, 32, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Van Velthoven, C.T.; Kavelaars, A.; van Bel, F.; Heijnen, C.J. Repeated mesenchymal stem cell treatment after neonatal hypoxia-ischemia has distinct effects on formation and maturation of new neurons and oligodendrocytes leading to restoration of damage, corticospinal motor tract activity, and sensorimotor function. J. Neurosci. 2010, 30, 9603–9611. [Google Scholar] [CrossRef] [PubMed]
- Colton, C.A. Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharmacol. 2009, 4, 399–418. [Google Scholar] [CrossRef] [PubMed]
- Gressens, P.; Marret, S.; Hill, J.M.; Brenneman, D.E.; Gozes, I.; Fridkin, M.; Evrard, P. Vasoactive intestinal peptide prevents excitotoxic cell death in the murine developing brain. J. Clin. Investig. 1997, 100, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Destot-Wong, K.D.; Liang, K.; Gupta, S.K.; Favrais, G.; Schwendimann, L.; Pansiot, J.; Baud, O.; Spedding, M.; Lelievre, V.; Mani, S.; et al. The ampa receptor positive allosteric modulator, s18986, is neuroprotective against neonatal excitotoxic and inflammatory brain damage through bdnf synthesis. Neuropharmacology 2009, 57, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Robertson, N.J.; Nakakeeto, M.; Hagmann, C.; Cowan, F.M.; Acolet, D.; Iwata, O.; Allen, E.; Elbourne, D.; Costello, A.; Jacobs, I. Therapeutic hypothermia for birth asphyxia in low-resource settings: A pilot randomised controlled trial. Lancet 2008, 372, 801–803. [Google Scholar] [CrossRef]
- Pauliah, S.S.; Shankaran, S.; Wade, A.; Cady, E.B.; Thayyil, S. Therapeutic hypothermia for neonatal encephalopathy in low- and middle-income countries: A systematic review and meta-analysis. PLoS ONE 2013, 8, e58834. [Google Scholar] [CrossRef] [PubMed]
- Bamji, M.S.; PV, V.S.M.; Williams, L.; Vardhana Rao, M.V. Maternal nutritional status & practices & perinatal, neonatal mortality in rural andhra pradesh, india. Indian J. Med. Res. 2008, 127, 44–51. [Google Scholar] [PubMed]
- Osredkar, D.; Thoresen, M.; Maes, E.; Flatebo, T.; Elstad, M.; Sabir, H. Hypothermia is not neuroprotective after infection-sensitized neonatal hypoxic-ischemic brain injury. Resuscitation 2014, 85, 567–572. [Google Scholar] [CrossRef] [PubMed]

| Mechanism | Clinical Trials | Pre-Clinical Studies |
|---|---|---|
| Endogenous | Erythropoietin, darbepoietin | Remote ischemic postconditioning |
| Stem cells | Endocannabinoids | |
| Melatonin | ||
| Exogenous | Monosialogangliosides | Argon |
| Xenon | Azithromycin | |
| Allopurinol | ||
| Topiramate | ||
| Magnesium sulfate |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, J.; Kumar, V.H.S. Current and Emerging Therapies in the Management of Hypoxic Ischemic Encephalopathy in Neonates. Children 2018, 5, 99. https://doi.org/10.3390/children5070099
Nair J, Kumar VHS. Current and Emerging Therapies in the Management of Hypoxic Ischemic Encephalopathy in Neonates. Children. 2018; 5(7):99. https://doi.org/10.3390/children5070099
Chicago/Turabian StyleNair, Jayasree, and Vasantha H.S. Kumar. 2018. "Current and Emerging Therapies in the Management of Hypoxic Ischemic Encephalopathy in Neonates" Children 5, no. 7: 99. https://doi.org/10.3390/children5070099
APA StyleNair, J., & Kumar, V. H. S. (2018). Current and Emerging Therapies in the Management of Hypoxic Ischemic Encephalopathy in Neonates. Children, 5(7), 99. https://doi.org/10.3390/children5070099





