Management of Congenital Heart Disease: State of the Art; Part I—ACYANOTIC Heart Defects
Abstract
:1. Introduction
2. Pulmonary Stenosis
2.1. Indications for Intervention
2.2. Timing of Intervention
2.3. Type of Intervention
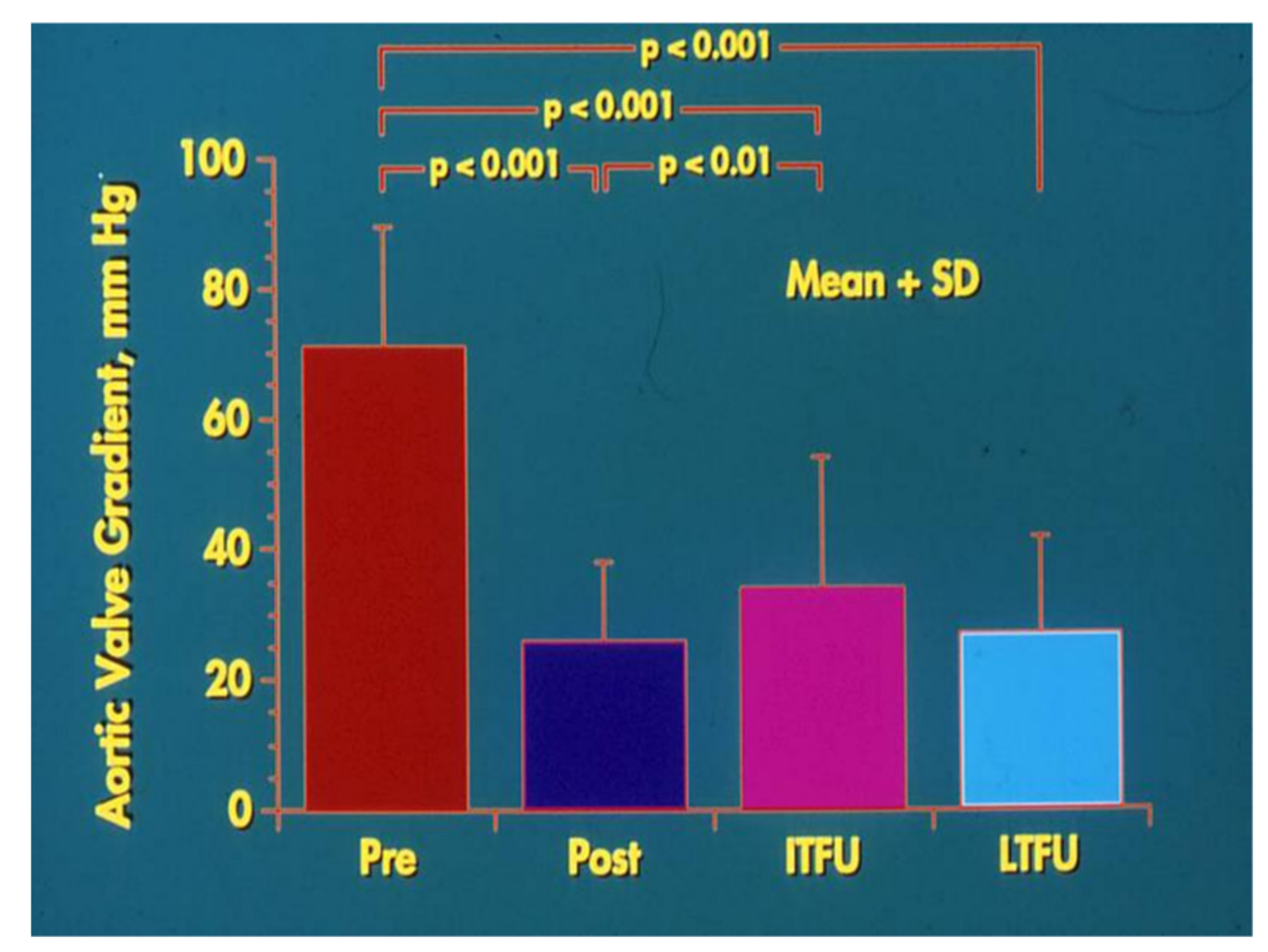
3. Aortic Stenosis
3.1. Indications for Intervention
3.2. Timing of Intervention
3.3. Type of Intervention
4. Coarctation of the Aorta
4.1. Indications for Intervention
4.2. Timing of Intervention
4.3. Type of Intervention
5. Atrial Septal Defect
5.1. Ostium Secundum ASDs
5.1.1. Indications for Intervention
5.1.2. Timing of Intervention
5.1.3. Type of Intervention
5.2. Ostium Primum ASDs
5.2.1. Indications for Intervention
5.2.2. Timing of Intervention
5.2.3. Type of Intervention
5.3. Sinus Venosus ASDs
5.4. Coronary Sinus ASDs
5.5. Patent Foramen Ovale
5.5.1. PFOs Associated with Other CHD
5.5.2. Residual PFOs in Previously Treated Complex CHD
5.5.3. PFOs Presumed to be the Seat of Paradoxical Embolism
5.5.4. PFOs in Platypnea-Orthodeoxia Syndrome
5.5.5. PFOs in Other Conditions
5.6. Type of Intervention
6. Ventricular Septal Defect (VSD)
6.1. Indications for Intervention
6.2. Timing of Intervention
6.3. Type of Intervention
6.3.1. CHF
6.3.2. Perimembranous VSDs
6.3.3. Supracristal VSDs
6.3.4. AV Septal VSDs
6.3.5. Muscular VSDs
6.3.6. Comments
7. Atrioventricular Septal Defect
7.1. Balanced AVSDs
7.1.1. Indications for Intervention
7.1.2. Timing of Intervention
7.1.3. Type of Intervention
7.2. Unbalanced AVSDs
7.2.1. Single-Ventricle Palliation (Fontan)
7.2.2. Biventricular Repair Along with Bidirectional Glenn Procedure
7.2.3. Biventricular Repair
7.2.4. Conversion from Single-Ventricle to Two-Ventricle Repair
8. Patent Ductus Arteriosus
8.1. Indications for Intervention
8.2. Timing of Intervention
8.3. Type of Intervention
8.4. PDA in the Premature
Conflicts of Interest
References
- Gross, R.E.; Hubbard, J.P. Surgical ligation of patent ductus arteriosus: A report of first successful case. J. Am. Med. Assoc. 1939, 112, 729–731. [Google Scholar] [CrossRef]
- Gibson, S.G. Cardiovascular system. In Brenneman’s Practice of Pediatrics; WF Prior Company: Hagerstown, MD, USA, 1945; pp. 1–99. [Google Scholar]
- Taussig, H.B. Congenital Malformations of the Heart; The Commonwealth Fund: New York, NY, USA, 1947; p. 618. [Google Scholar]
- Rubio-Alvarez, V.; Limon-Lason, R.; Soni, L. Valvalotomias intracardiacas por medico de un cateter. Arch. Inst. Cardiol. Mexico 1953, 23, 183–192. [Google Scholar] [PubMed]
- Rubio, V.; Limon-Lason, R. Treatment of pulmonary valve stenosis and of tricuspid valve stenosis using a modified catheter. In Proceedings of the Second World Congress of Cardiology, Washington, DC, USA, 12–17 September 1954. [Google Scholar]
- Rao, P.S. Interventional pediatric cardiology: State of the art and future directions. Pediat. Cardiol. 1998, 19, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S. Diagnosis and management of acyanotic heart disease: Part I—Obstructive lesions. Indian J. Pediatr. 2005, 72, 496–502. [Google Scholar] [CrossRef]
- Rao, P.S. Diagnosis and management of acyanotic heart disease: Part II—Left-to-right shunt lesions. Indian J. Pediatr. 2005, 72, 503–512. [Google Scholar] [CrossRef]
- Rao, P.S. Principles of management of the neonate with congenital heart disease. Neonatol. Today 2007, 2, 1–10. [Google Scholar]
- Rao, P.S. Diagnosis and management of cyanotic congenital heart disease: Part I. Indian J. Pediatr. 2009, 76, 57–70. [Google Scholar] [CrossRef]
- Rao, P.S. Diagnosis and management of cyanotic congenital heart disease: Part II. Indian J. Pediatr. 2009, 76, 297–308. [Google Scholar] [CrossRef]
- Rao, P.S. Congenital heart defects—A review. In Congenital Heart Disease—Selected Aspects; Rao, P.S., Ed.; InTech: Rijeka, Croatia, 2012; pp. 3–44, Chapter 1; ISBN 978-953-307-472-6. [Google Scholar]
- Rao, P.S. Consensus on timing of intervention for common congenital heart diseases: Part I—Acyanotic heart defects. Indian J. Pediatr. 2013, 80, 72–78. [Google Scholar] [CrossRef]
- Rao, P.S. Consensus on timing of intervention for common congenital heart diseases: Part II—Cyanotic heart defects. Indian J. Pediatr. 2013, 80, 663–674. [Google Scholar] [CrossRef]
- Rao, P.S.; Harris, A.D. Recent advances in managing septal defects: Atrial septal defects. F1000 Faculty Rev 2017, 6, 2042. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S.; Harris, A.D. Recent advances in managing septal defects: Ventricular septal defects and atrioventricular septal defects. F1000Research 2018, 7, F1000. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S. Pulmonary valve disease. In Valvular Heart Disease, 3rd ed.; Alpert, J.S., Dalen, J.E., Rahimtoola, S., Eds.; Lippincott Raven: Philadelphia, PA, USA, 2000; pp. 339–376. [Google Scholar]
- Nugent, E.W.; Freedom, R.M.; Nora, J.J.; Ellison, R.C.; Rowe, R.D.; Nadas, A.S. Clinical course of pulmonic stenosis. Circulation 1977, 56, 18–47. [Google Scholar]
- Rao, P.S. Indications for balloon pulmonary valvuloplasty (editorial). Am. Heart J. 1988, 116, 1661–1662. [Google Scholar] [CrossRef]
- Jureidini, S.B.; Rao, P.S. Critical pulmonary stenosis in the neonate: Role of transcatheter management. J. Invasive Cardiol. 1996, 8, 326–331. [Google Scholar] [PubMed]
- Rao, P.S. Role of interventional cardiology in neonates: Part II—Balloon angioplasty/valvuloplasty. Congenit. Cardiol. Today 2008, 6, 1–14. [Google Scholar]
- Rao, P.S. Percutaneous balloon pulmonary valvuloplasty: State of the art. Catheter. Cardiovasc. Interv. 2007, 69, 747–763. [Google Scholar] [CrossRef]
- Rao, P.S. Influence of balloon size on the short-term and long-term results of balloon pulmonary valvuloplasty. Tex. Heart Inst. J. 1987, 14, 57–61. [Google Scholar]
- Rao, P.S. Late pulmonary insufficiency after balloon dilatation of the pulmonary valve (Letter). Catheter. Cardiovasc. Interv. 2000, 49, 118–119. [Google Scholar]
- Rao, P.S. How big a balloon and how many balloons for pulmonary valvuloplasty? (editorial) Am. Heart J. 1988, 116, 577–580. [Google Scholar] [CrossRef]
- Rao, P.S.; Fawzy, M.E. Double balloon technique for percutaneous balloon pulmonary valvuloplasty: Comparison with single balloon technique. J. Interv. Cardiol. 1988, 1, 257–262. [Google Scholar] [CrossRef]
- Rao, P.S.; Galal, O.; Patnana, M.; Buck, S.H.; Wilson, A.D. Results of three-to-ten-year follow-up of balloon dilatation of the pulmonary valve. Heart 1998, 80, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.K.; Rao, P.S. Left heart outflow obstructions. In Cardiology, 3rd ed.; Crawford, M.H., DiMarco, J.P., Paulus, W.J., Eds.; Mosby Elsevier: Edinburgh, UK, 2010; pp. 1507–1518. ISBN 978-0-7234-3485-6. [Google Scholar]
- Rao, P.S. Balloon aortic valvuloplasty: A review. Clin. Cardiol. 1990, 13, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S. Balloon aortic valvuloplasty. J. Interv. Cardiol. 1998, 11, 319–329. [Google Scholar] [CrossRef]
- Agu, N.C.; Rao, P.S. Balloon aortic valvuloplasty. Pediatr. Ther. 2012, 5, 004. [Google Scholar] [CrossRef]
- Rao, P.S. Long-term follow-up results after balloon dilatation of pulmonic stenosis, aortic stenosis and coarctation of the aorta: A review. Progr. Cardiovasc. Dis. 1999, 42, 59–74. [Google Scholar] [CrossRef]
- Rao, P.S. Balloon aortic valvuloplasty (Editorial). Indian Heart J. 2016, 68, 592–595. [Google Scholar] [CrossRef]
- Rao, P.S. Coarctation of the aorta. In Secondary Forms of Hypertension; Ram, C.V.S., Ed.; W.B. Saunders: Philadelphia, PA, USA, 1995; Volume 15, pp. 81–105. [Google Scholar]
- Rao, P.S. Balloon angioplasty of native aortic coarctation. In Transcatheter Therapy in Pediatric Cardiology; Rao, P.S., Ed.; Wiley-Liss: New York, NY, USA, 1993; pp. 153–196. [Google Scholar]
- Rao, P.S. Coarctation of the aorta. Curr. Cardiol. Rep. 2005, 7, 425–434. [Google Scholar] [CrossRef]
- Doshi, A.R.; Rao, P.S. Coarctation of aorta—Management options and decision making. Pediatr. Ther. 2012, 5, 006. [Google Scholar] [CrossRef]
- Rao, P.S.; Wilson, A.D.; Brazy, J. Transumbilical balloon coarctation angioplasty in a neonate with critical aortic coarctation. Am. Heart J. 1992, 124, 1622–1624. [Google Scholar] [CrossRef]
- Salahuddin, N.; Wilson, A.D.; Rao, P.S. An unusual presentation of coarctation of the aorta in infancy: Role of balloon angioplasty in the critically ill infant. Am. Heart J. 1991, 122, 1772–1775. [Google Scholar] [CrossRef]
- Rao, P.S. Should balloon angioplasty be used as a treatment of choice for native aortic coarctations? J. Invasive Cardiol. 1996, 8, 301–313. [Google Scholar] [PubMed]
- Rao, P.S. Balloon angioplasty of aortic recoarctation following previous surgery. In Transcatheter Therapy in Pediatric Cardiology; Rao, P.S., Ed.; Wiley-Liss: New York, NY, USA, 1993; pp. 197–212. [Google Scholar]
- Rao, P.S.; Wilson, A.D.; Chopra, P.S. Immediate and follow-up results of balloon angioplasty of postoperative recoarctation in infants and children. Am. Heart J. 1990, 120, 1315–1320. [Google Scholar] [CrossRef]
- Siblini, G.; Rao, P.S.; Nouri, S.; Ferdman, B.; Jureidini, S.B.; Wilson, A.D. Long-term follow-up results of balloon angioplasty of postoperative aortic recoarctation. Am. J. Cardiol. 1998, 81, 61–67. [Google Scholar] [CrossRef]
- Rao, P.S.; Galal, O.; Wilson, A.D. Feasibility and effectiveness of repeat balloon dilatation of restenosed obstruction following previous balloon valvuloplasty/angioplasty. Am. Heart J. 1966, 132, 403–407. [Google Scholar]
- Rao, P.S. Transcatheter interventions in critically ill neonates and infants with aortic coarctation. Ann. Pediatr. Card 2009, 2, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Mullins, C.E. Inappropriate stents: Primary cause of failure of stent redilation in coarctation of the aorta. Catheter. Cardiovasc. Interv. 2008, 72, 557–558. [Google Scholar] [CrossRef]
- Rao, P.S. Stents in the management of aortic coarctation in young children (Editorial). J. Am. Coll. Cardiol. 2009, 2, 884–886. [Google Scholar] [CrossRef]
- Shepherd, E.; Connolly, G.M.; Morgan, G. Using the Valeo dilatable stent in coarctation stenting for small children: Expanding the inclusion criteria for coarctation stenting? BMJ Case Rep. 2013, 2013, 202095. [Google Scholar] [CrossRef]
- Qureshi, S.A.; Zubrzycka, M.; Brzezinska-Rajszys, G. Use of covered Cheatham-Platinum stents in aortic coarctation and recoarctation. Cardiol. Young 2004, 14, 50–54. [Google Scholar] [CrossRef]
- Rao, P.S. Percutaneous management of aortic coarctation. In Cardiac Catheterization and Imaging (From Pediatrics to Geriatrics); Vijayalakshmi, I.B., Ed.; Jaypee Publications: New Delhi, India, 2015; pp. 433–471. [Google Scholar]
- Rao, P.S. Atrial septal defect—A review. In Atrial Septal Defect; Rao, P.S., Ed.; InTech: Rijeka, Croatia, 2012; pp. 3–20. ISBN 978-953-51-0531-2. [Google Scholar]
- Rao, P.S. When and how should atrial septal defects be closed in adults. J. Invasive Cardiol. 2009, 21, 76–82. [Google Scholar] [PubMed]
- Rao, P.S. Why, When and how should atrial septal defects be closed in adults. In Atrial Septal Defect; Rao, P.S., Ed.; InTech: Rijeka, Croatia, 2012; pp. 121–138. ISBN 978-953-51-0531-2. [Google Scholar]
- King, T.D.; Thompson, S.L.; Steiner, C.; Mills, N.L. Secundum atrial septal defect: Nonoperative closure during cardiac catheterization. JAMA 1976, 235, 2506–2509. [Google Scholar] [CrossRef] [PubMed]
- Rashkind, W.J.; Cuaso, C.E. Transcatheter closure of atrial septal defects in children. Eur. J. Cardiol. 1977, 8, 119–112. [Google Scholar]
- Chopra, P.S.; Rao, P.S. History of the development of atrial septal occlusion devices. Curr. Interv. Cardiol. Rep. 2000, 2, 63–69. [Google Scholar] [PubMed]
- Rao, P.S. History of atrial septal occlusion devices. In Catheter Based Devices in the Treatment of Non-Coronary Cardiovascular Disease in Adults and Children; Rao, P.S., Kern, M.J., Eds.; Lippincott, William & Wilkins: Philadelphia, PA, USA, 2003; pp. 1–9. [Google Scholar]
- Alapati, S.; Rao, P.S. Historical aspects of transcatheter occlusion of atrial septal defects. In Atrial Septal Defect; Rao, P.S., Ed.; InTech: Rijeka, Croatia, 2012; pp. 57–84. ISBN 978-953-51-0531-2. [Google Scholar]
- Rao, P.S. History of transcatheter interventions in pediatric cardiology. In Cardiac Catheterization and Imaging (From Pediatrics to Geriatrics); Vijayalakshmi, I.B., Ed.; Jaypee Publications: New Delhi, India, 2015; pp. 3–20. [Google Scholar]
- Berger, F.; Vogel, M.; Alexi-Meskishvili, V.; Lange, P.E. Comparison of results and complications of surgical and Amplatzer device closure of atrial septal defects. J. Thorac. Cardiovasc. Surg. 1999, 118, 674–678. [Google Scholar] [CrossRef]
- Du, Z.D.; Hijazi, Z.M.; Kleinman, C.S. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: Results of multicenter nonrandomized trial. J. Am. Coll. Cardiol. 2002, 39, 1836–1844. [Google Scholar] [CrossRef]
- Kim, J.J.; Hijazi, Z.M. Clinical outcomes and costs of Amplatzer transcatheter closure as compared with surgical closure of ostium secundum atrial septal defects. Med. Sci. Monit. 2002, 12, CR787–CR791. [Google Scholar] [CrossRef]
- Bettencourt, N.; Salome, N.; Carneiro, F.; Gonçalves, M.; Ribeiro, J.; Braga, J.P.; Fonseca, C.; Correia, D.M.; Vouga, L.; Ribeiro, V.G. Atrial septal closure in adults: Surgery versus Amplatzer—Comparison of results. Rev. Port. Cardiol. 2003, 22, 1203–1211. [Google Scholar]
- Rao, P.S. Catheter closure of atrial septal defects. J. Invasive Cardiol. 2003, 15, 398–400. [Google Scholar]
- Minich, L.L.; Atz, A.M.; Colan, S.D.; Sleeper, L.A.; Mital, S.; Jaggers, J.; Margossian, R.; Prakash, A.; Li, J.S.; Cohen, M.S.; Lacro, R.V.; Klein, G.L.; Hawkins, J.A. Partial and transitional atrioventricular septal defect outcomes. Ann. Thorac. Surg. 2010, 89, 530–536. [Google Scholar] [CrossRef]
- Devlin, P.J.; Backer, C.L.; Eltayeb, O.; Mongé, M.C.; Hauck, A.L.; Costello, J.M. Repair of partial atrioventricular septal defect: Age and outcomes. Ann. Thorac. Surg. 2016, 102, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Krupickova, S.; Morgan, G.J.; Cheang, M.H.; Rigby, M.L.; Franklin, R.C.; Battista, A.; Spanaki, A.; Bonello, B.; Ghez, O.; Anderson, D.; et al. Symptomatic partial and transitional atrioventricular septal defect repaired in infancy. Heart 2018, 104, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Konstantinov, I.E.; Buratto, E. Repair of partial atrioventricular septal defects in infancy: A paradigm shift or a road block? Heart 2018, 104, 1388–1389. [Google Scholar] [CrossRef] [PubMed]
- Di Bernardo, S.; Fasnacht, M.; Berger, F. Transcatheter closure of a coronary sinus defect with an Amplatzer septal occluder. Catheter. Cardiovasc. Interv. 2003, 60, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S. Perinatal circulatory physiology. Indian J. Pediatr. 1991, 58, 441–451. [Google Scholar] [PubMed]
- Rao, P.S. Fetal and neonatal circulation. In Cardiac Anesthesia for Infants and Children; Kambam, J., Ed.; Mosby-Year Book: St. Louis, MO, USA, 1994; pp. 10–19, Chapter 2. [Google Scholar]
- Rao, P.S. Perinatal circulatory physiology: It’s influence on clinical manifestations of neonatal heart disease—Part I. Neonatol. Today 2008, 3, 6–12. [Google Scholar]
- Rao, P.S. Role of interventional cardiology in neonates: Part I. Non-surgical atrial septostomy. Congenit. Cardiol. Today 2007, 5, 1–12. [Google Scholar]
- Rao, P.S.; Chandar, J.S.; Sideris, E.B. Role of inverted buttoned device in trans-catheter occlusion of atrial septal defect or patent foramen ovale with right-to-left shunting associated with previously operated complex congenital cardiac anomalies. Am. J. Cardiol. 1997, 80, 914–921. [Google Scholar] [CrossRef]
- Ende, D.J.; Chopra, P.S.; Rao, P.S. Transcatheter closure of atrial septal defect or patent foramen ovale with the buttoned device for prevention of recurrence of paradoxic embolism. Am. J. Cardiol. 1996, 78, 233–236. [Google Scholar] [CrossRef]
- Rao, P.S.; Palacios, I.F.; Bach, R.G.; Bitar, S.R.; Sideris, E.B. Platypnea-orthodeoxia syndrome: Management by trans-catheter buttoned device implantation. Catheter. Cardiovasc. Interv. 2001, 54, 77–82. [Google Scholar] [CrossRef]
- Bitar, S.; Rao, P.S. Platypnea-orthodeoxia syndrome: Transcatheter management. In Catheter Based Devices for Treatment of Noncoronary Cardiovascular Disease in Adults and Children; Rao, P.S., Kern, M.J., Eds.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2003; pp. 129–132, Chapter 15. [Google Scholar]
- Rao, P.S. Transcatheter management of platypnea-orthodeoxia syndrome (Editorial). J. Invasive Cardiol. 2004, 16, 583–584. [Google Scholar] [PubMed]
- Fu, Y.C.; Bass, J.; Amin, Z.; Radtke, W.; Cheatham, J.P.; Hellenbrand, W.E.; Balzer, D.; Cao, Q.L.; Hijazi, Z.M. Transcatheter closure of perimembranous ventricular septal defects using the new Amplatzer membranous VSD occluder: Results of the U.S. phase I trial. J. Am. Coll. Cardiol. 2006, 47, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S. Perimembranous ventricular septal defect closure with the Amplatzer device. J. Invasive Cardiol. 2008, 20, 217–218. [Google Scholar] [PubMed]
- Rao, P.S. Absorbable pulmonary artery band in tricuspid atresia (Editorial). Ann. Thorac. Surg. 2001, 71, 361–362. [Google Scholar] [CrossRef]
- Holzer, R.; Balzer, D.; Cao, Q.L.; Lock, K.; Hijazi, Z.M. Device closure of muscular ventricular septal defects using the Amplatzer muscular ventricular septal defect occluder: Immediate and mid-term results of a U.S. registry. J. Am. Coll. Cardiol. 2004, 43, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Bacha, E.A.; Cao, Q.L.; Starr, J.P.; Waight, D.; Ebeid, M.R.; Hijazi, Z.M. Perventricular device closure of muscular ventricular septal defects on the beating heart: Technique and results. J. Thorac. Cardiovasc. Surg. 2003, 126, 1718–1723. [Google Scholar] [CrossRef]
- Amin, Z.; Danford, D.A.; Lof, J.; Duncan, K.F.; Froemming, S. Intraoperative device closure of perimembranous ventricular septal defects without cardiopulmonary bypass: Preliminary results with the perventricular technique. J. Thorac. Cardiovasc. Surg. 2004, 127, 234–241. [Google Scholar] [CrossRef]
- Xing, Q.; Pan, S.; An, Q. Minimally invasive perventricular device closure of perimembranous ventricular septal defect without cardiopulmonary bypass: Multicenter experience and mid-term follow-up. J. Thorac. Cardiovasc. Surg. 2010, 139, 1409–1415. [Google Scholar] [CrossRef]
- Michel-Behnke, I.; Ewert, P.; Koch, A. Device closure of ventricular septal defects by hybrid procedures: A multicenter retrospective study. Catheter. Cardiovasc. Interv. 2011, 77, 242–251. [Google Scholar] [CrossRef]
- Cooney, T.P.; Thurlbeck, W.M. Pulmonary hypoplasia in Down’s syndrome. N. Engl. J. Med. 1982, 307, 1170–1173. [Google Scholar] [CrossRef]
- Hals, J.; Hagemo, P.S.; Thaulow, E.; Sørland, S.J. Pulmonary vascular resistance in complete atrioventricular septal defect. A comparison between children with and without Down’s syndrome. Acta Paediatr. 1993, 82, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Fan, Q.; Iwase, T.; Hirata, Y.; An, Q. Modified single-patch technique versus two patch technique for the repair of complete atrioventricular septal defect: A meta-analysis. Pediatr. Cardiol. 2017, 38, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S. Fontan operation: Indications, short and long term outcomes. Indian J. Pediatr. 2015, 82, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Delmo Walter, E.M.; Ewert, P.; Hetzer, R.; Hubler, M.; Alexi-Meskishvili, V.; Lange, P.; Berger, F. Biventricular repair in children with complete atrioventricular septal defect and a small left ventricle. Eur. J. Cardiothorac. Surg. 2008, 33, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Emani, S.M.; McElhinney, D.B.; Tworetzky, W.; Myers, P.O.; Schroeder, B.; Zurakowski, D.; Pigula, F.A.; Marx, G.R.; Lock, J.E.; del Nido, P.J. Staged left ventricular recruitment after single-ventricle palliation in patients with borderline left heart hypoplasia. J. Am. Coll. Cardiol. 2012, 60, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Nathan, M.; Liu, H.; Pigula, F.A.; Fynn-Thompson, F.; Emani, S.; Baird, C.A.; Marx, G.; Mayer, J.E.; Del Nido, P.J. Biventricular conversion after single-ventricle palliation in unbalanced atrioventricular canal defects. Ann. Thorac. Surg. 2013, 95, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S. Perinatal circulatory physiology. In Perinatal Cardiology: A Multidisciplinary Approach; Rao, P.S., Vidyasagar, D., Eds.; Cardiotext Publishing: Minneapolis, MN, USA, 2015; pp. 1–15, Chapter 1. [Google Scholar]
- Krichenko, A.; Benson, L.; Burrows, P.; Möes, C.A.; McLaughlin, P.; Freedom, R.M. Angiographic classification of the isolated persistently patent ductus arteriosus and implications for percutaneous catheter occlusion. Am. J. Cardiol. 1989, 63, 887–889. [Google Scholar] [CrossRef]
- Naidu, D.P.; Breinholt, J.P., III; Rao, P.S. Patent ductus arteriosus. In Essentials of Neonatal Ventilation; Rajiv, P.K., Lakshminrusimha, S., Vidyasagar, D., Eds.; Elsevier: Gurgaon, India, 2006. [Google Scholar]
- Rao, P.S. Transcatheter occlusion of patent ductus arteriosus: Which method to use and which ductus to close? (Editorial). Am. Heart J. 1996, 132, 905–909. [Google Scholar]
- Rao, P.S. Coil occlusion of patent ductus arteriosus (editorial). J. Invasive Cardiol. 2001, 13, 36–38. [Google Scholar]
- Rao, P.S. Transcatheter closure of moderate-to-large patent ductus arteriosus (Editorial). J. Invasive Cardiol. 2001, 13, 303–306. [Google Scholar]
- Rao, P.S. Percutaneous closure of patent ductus arteriosus: State of the art (Editorial). J. Invasive Cardiol. 2007, 19, 299–302. [Google Scholar] [PubMed]
- Rao, P.S. Percutaneous closure of patent ductus arteriosus—Current status. J. Invasive Cardiol. 2011, 23, 517–20. [Google Scholar] [PubMed]
- Yarrabolu, T.R.; Rao, P.S. Transcatheter closure of patent ductus arteriosus. Pediatr. Ther. 2012, 5, 005. [Google Scholar] [CrossRef]
- Laborde, F.; Noirhomme, P.; Karam, J. A new video-assisted thoracoscopic surgical technique for interruption of patient ductus arteriosus in infants and children. J. Thorac. Cardiovasc. Surg. 1993, 105, 278–280. [Google Scholar] [PubMed]
- Hines, M.H. Video assisted thoracoscopic surgical (VATS) closure of the patent ductus arteriosus in premature and term newborn infants. In Perinatal Cardiology: A Multidisciplinary Approach; Rao, P.S., Vidyasagar, D., Eds.; Cardiotext Publishing: Minneapolis, MN, USA, 2015; Chapter 40. [Google Scholar]
- Porstmann, W.; Wierny, L.; Warnke, H. Der Verschluβ des Ductus arteriosus persistens ohne Thorakotomie (Vorläufige, Mitteilung). Thoraxchirurgie 1967, 15, 199–203. [Google Scholar]
- Rashkind, W.J.; Cuaso, C.C. Transcatheter closure of a patent ductus arteriosus: Successful use in a 3.5kg infant. Pediatr. Cardiol. 1979, 1, 3–7. [Google Scholar] [CrossRef]
- Rao, P.S. Summary and comparison of patent ductus arteriosus closure devices. Curr. Interv. Cardiol. Rep. 2001, 3, 268–274. [Google Scholar] [PubMed]
- Rao, P.S. History of transcatheter patent ductus arteriosus closure devices. In Catheter Based Devices for the Treatment of Noncoronary Cardiovascular Disease in Adults and Children; Rao, P.S., Kern, M.J., Eds.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2003; pp. 145–153. [Google Scholar]
- Rao, P.S. Historical aspects of transcatheter treatment of heart disease in children. Pediatr. Ther. 2012, 5, 002. [Google Scholar] [CrossRef]
- Rao, P.S. Summary and comparison of patent ductus arteriosus closure methods. In Catheter Based Devices for Treatment of Noncoronary Cardiovascular Disease in Adults and Children; Rao, P.S., Kern, M.J., Eds.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2003; pp. 219–228, Chapter 25. [Google Scholar]












© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, P.S. Management of Congenital Heart Disease: State of the Art; Part I—ACYANOTIC Heart Defects. Children 2019, 6, 42. https://doi.org/10.3390/children6030042
Rao PS. Management of Congenital Heart Disease: State of the Art; Part I—ACYANOTIC Heart Defects. Children. 2019; 6(3):42. https://doi.org/10.3390/children6030042
Chicago/Turabian StyleRao, P. Syamasundar. 2019. "Management of Congenital Heart Disease: State of the Art; Part I—ACYANOTIC Heart Defects" Children 6, no. 3: 42. https://doi.org/10.3390/children6030042
APA StyleRao, P. S. (2019). Management of Congenital Heart Disease: State of the Art; Part I—ACYANOTIC Heart Defects. Children, 6(3), 42. https://doi.org/10.3390/children6030042




