Sinusoidal Obstruction Syndrome Following Myeloablative Therapy and Tranexamic Acid Treatment for Hemorrhage in Two Patients with Neuroblastoma
Abstract
1. Introduction
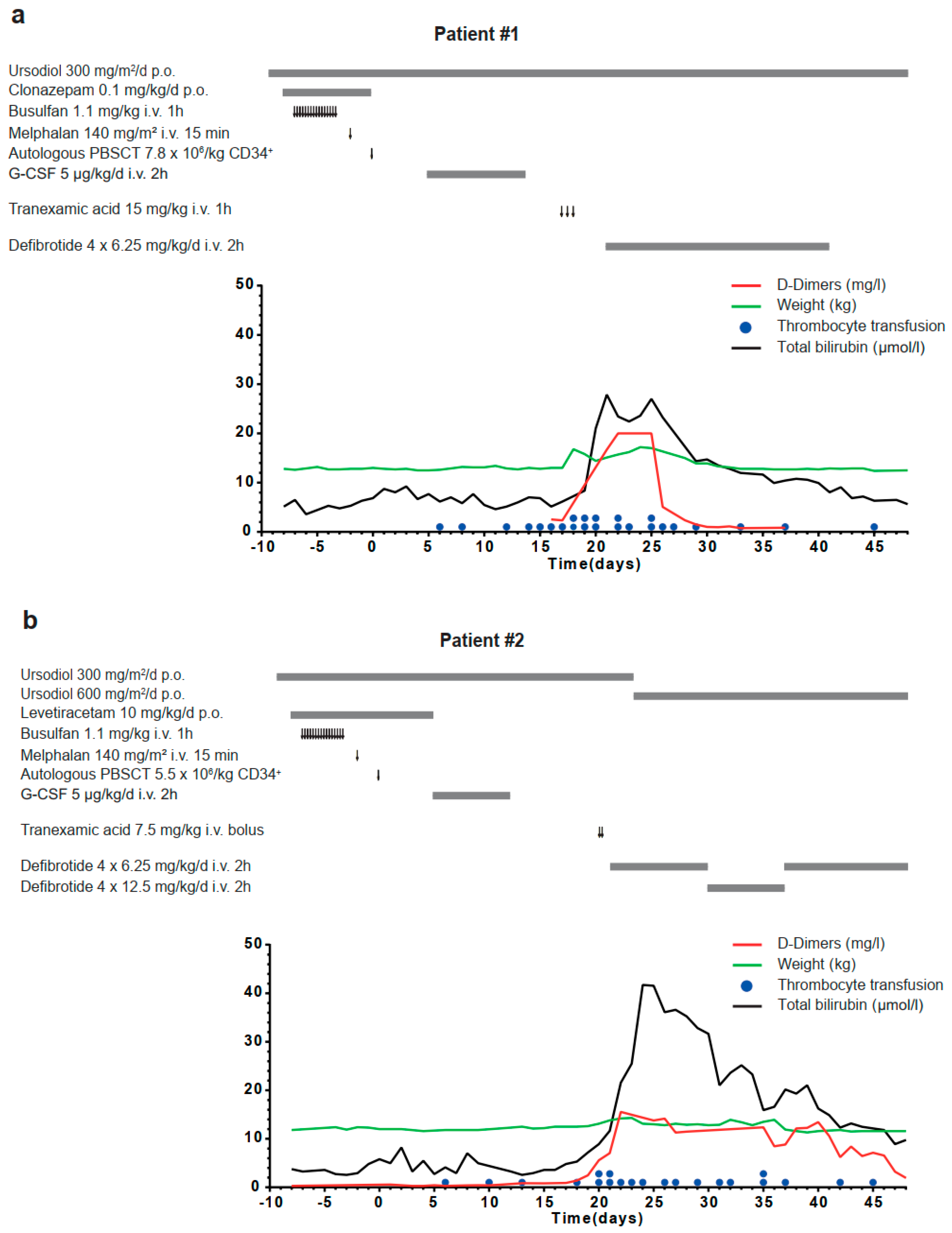
2. Results
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohty, M.; Malard, F.; Abecassis, M.; Aerts, E.; Alaskar, A.S.; Aljurf, M.; Arat, M.; Bader, P.; Baron, F.; Bazarbachi, A.; et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: A new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016, 51, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Coppell, J.A.; Brown, S.A.; Perry, D.J. Veno-occlusive disease: Cytokines, genetics, and haemostasis. Blood Rev. 2003, 17, 63–70. [Google Scholar] [CrossRef]
- Jacobs, P.; Miller, J.L.; Uys, C.J.; Dietrich, B.E. Fatal veno-occlusive disease of the liver after chemotherapy, whole-body irradiation and bone marrow transplantation for refractory acute leukaemia. S. Afr. Med. J. 1979, 55, 5–10. [Google Scholar] [PubMed]
- Berk, P.D.; Popper, H.; Krueger, G.R.; Decter, J.; Herzig, G.; Graw, R.G., Jr. Veno-occlusive disease of the liver after allogeneic bone marrow transplantation: Possible association with graft-versus-host disease. Ann. Intern. Med. 1979, 90, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.J.; Lee, K.S.; Beschorner, W.E.; Vogel, V.G.; Grochow, L.B.; Braine, H.G.; Vogelsang, G.B.; Sensenbrenner, L.L.; Santos, G.W.; Saral, R. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation 1987, 44, 778–783. [Google Scholar] [CrossRef] [PubMed]
- McDonald, G.B.; Sharma, P.; Matthews, D.E.; Shulman, H.M.; Thomas, E.D. Venocclusive disease of the liver after bone marrow transplantation: Diagnosis, incidence, and predisposing factors. Hepatology 1984, 4, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Corbacioglu, S.; Carreras, E.; Ansari, M.; Balduzzi, A.; Cesaro, S.; Dalle, J.H.; Dignan, F.; Gibson, B.; Guengoer, T.; Gruhn, B.; et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: A new classification from the European society for blood and marrow transplantation. Bone Marrow Transplant. 2018, 53, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Samson, A.L.; Alwis, I.; Maclean, J.A.A.; Priyananda, P.; Hawkett, B.; Schoenwaelder, S.M.; Jackson, S.P. Endogenous fibrinolysis facilitates clot retraction in vivo. Blood 2017, 130, 2453–2462. [Google Scholar] [CrossRef] [PubMed]
- Badenoch, A.; Sharma, A.; Gower, S.; Selzner, M.; Srinivas, C.; Wąsowicz, M.; McCluskey, S.A. The Effectiveness and Safety of Tranexamic Acid in Orthotopic Liver Transplantation Clinical Practice: A Propensity Score Matched Cohort Study. Transplantation 2017, 101, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.; Shakur, H.; Afolabi, A.; Brohi, K.; Coats, T.; Dewan, Y.; Gando, S.; Guyatt, G.; Hunt, B.J.; Morales, C.; et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: An exploratory analysis of the CRASH-2 randomised controlled trial. Lancet 2011, 377, 1096–1101, 1101.e1–1101.e2. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; An, L.; Fang, T.; Pan, B.; Sun, H.; Chen, C.; Cao, J.; Li, Z.; Xu, K. A murine model of hepatic veno-occlusive disease induced by allogeneic hematopoietic stem cell transplantation. Cell Biochem. Biophys. 2013, 67, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Palek, R.; Liska, V. A critical analysis of experimental animal models of sinusoidal obstruction syndrome. J. Clin. Exp. Hepatol. 2019, 9, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Aisa, Y.; Shimizu, T.; Yamazaki, R.; Mihara, A.; Yajima, T.; Hibi, T.; Ikeda, Y.; Okamoto, S. Hepatic veno-occlusive disease after tranexamic acid administration in patients undergoing allogeneic hematopoietic stem cell transplantation. Am. J. Hematol. 2007, 82, 838–839. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Kreissman, S.G.; London, W.B.; Naranjo, A.; Cohn, S.L.; Hogarty, M.D.; Tenney, S.C.; Haas-Kogan, D.; Shaw, P.J.; Geiger, J.D.; et al. A phase III randomized clinical trial (RCT) of tandem myeloablative autologous stem cell transplant (ASCT) using peripheral blood stem cell (PBSC) as consolidation therapy for high-risk neuroblastoma (HR-NB): A Children’s Oncology Group (COG) study. J. Clin. Oncol. 2016, 34, LBA3. [Google Scholar] [CrossRef]
| Baltimore Criteria for Adults, 1987 [5] | Modified Seattle Criteria for Adults, 1984 [6] | EMBT Criteria for Children, 2018 [7] |
|---|---|---|
Bilirubin serum level >2 mg/dL and at least 2 of following within 20 days of transplant:
| Two of following occurring within 21 days after HSCT:
| No limitation for time of SOS onset The presence of two or more of the following:
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zirngibl, F.; Flemmig, C.; Lang, P.; Künkele, A.; Eggert, A.; Schulte, J.H.; Deubzer, H.E. Sinusoidal Obstruction Syndrome Following Myeloablative Therapy and Tranexamic Acid Treatment for Hemorrhage in Two Patients with Neuroblastoma. Children 2020, 7, 198. https://doi.org/10.3390/children7110198
Zirngibl F, Flemmig C, Lang P, Künkele A, Eggert A, Schulte JH, Deubzer HE. Sinusoidal Obstruction Syndrome Following Myeloablative Therapy and Tranexamic Acid Treatment for Hemorrhage in Two Patients with Neuroblastoma. Children. 2020; 7(11):198. https://doi.org/10.3390/children7110198
Chicago/Turabian StyleZirngibl, Felix, Carina Flemmig, Peter Lang, Annette Künkele, Angelika Eggert, Johannes H. Schulte, and Hedwig E. Deubzer. 2020. "Sinusoidal Obstruction Syndrome Following Myeloablative Therapy and Tranexamic Acid Treatment for Hemorrhage in Two Patients with Neuroblastoma" Children 7, no. 11: 198. https://doi.org/10.3390/children7110198
APA StyleZirngibl, F., Flemmig, C., Lang, P., Künkele, A., Eggert, A., Schulte, J. H., & Deubzer, H. E. (2020). Sinusoidal Obstruction Syndrome Following Myeloablative Therapy and Tranexamic Acid Treatment for Hemorrhage in Two Patients with Neuroblastoma. Children, 7(11), 198. https://doi.org/10.3390/children7110198







