Opioid-Induced Respiratory Depression in Pediatric Palliative Care Patients with Severe Neurological Impairment—A Scoping Literature Review and Case Reports
Abstract
1. Introduction
2. Materials and Methods
3. Results
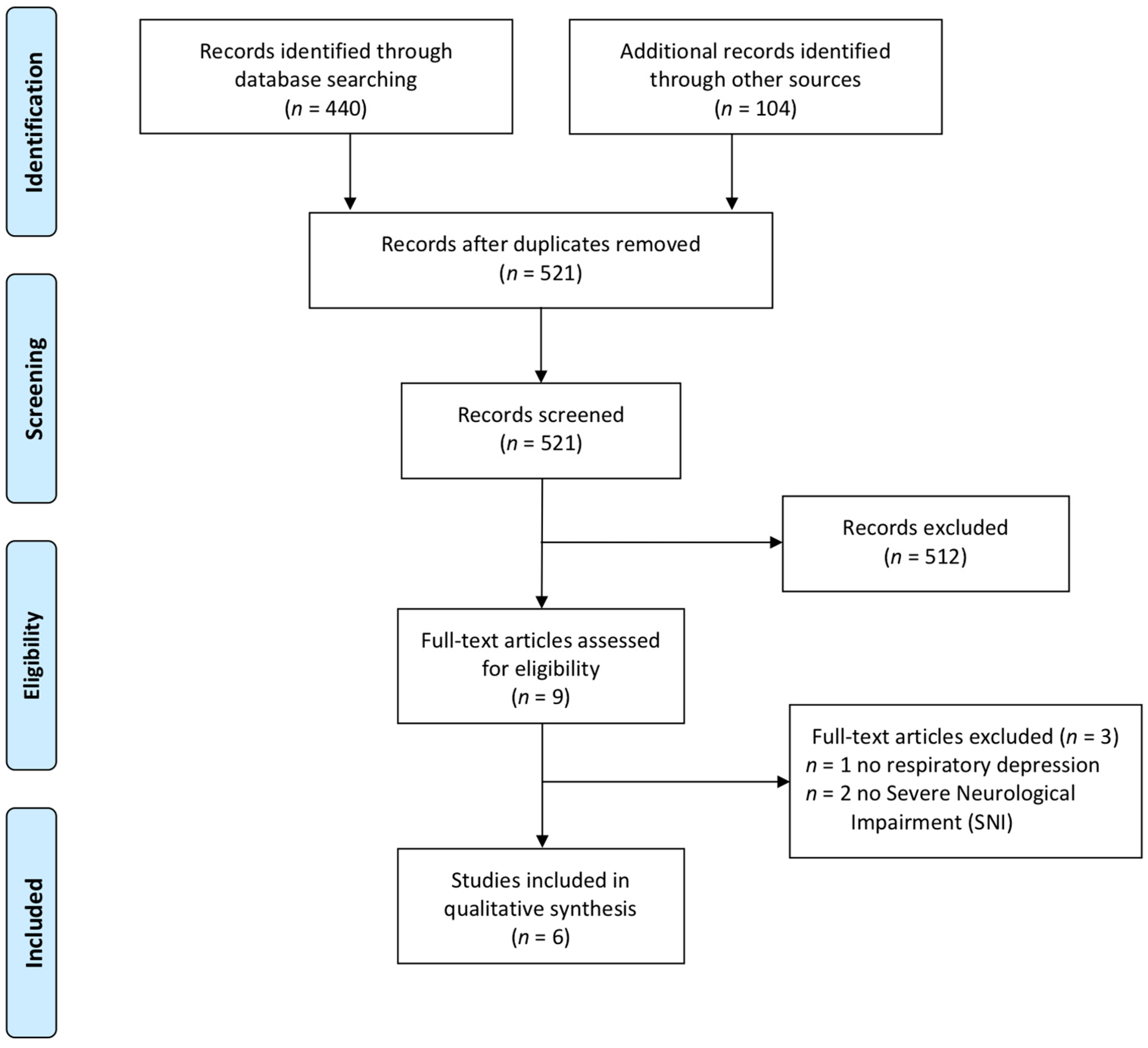
3.1. Selection of Sources of Evidence
3.2. Incidence of Opioid-Induced Respiratory Depression Events in Children
3.3. Risk factors of OIRD
3.3.1. Severe Neurological Impairment
3.3.2. Polypharmacy, Comorbidities, and Additional Risk Factors
3.3.3. Preventive Strategies
3.4. Case Reports
3.5. General Information
3.5.1. Case 1
3.5.2. Case 2
3.5.3. Case 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siden, H. Pediatric Palliative Care for Children with Progressive Non-Malignant Diseases. Children 2018, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, J.A.; Russell, S.; DeWitt, P.E.; Feudtner, C.; Dai, D.; Bennett, T.D. R Package for Pediatric Complex Chronic Condition Classification. JAMA Pediatr. 2018, 172, 596. [Google Scholar] [CrossRef] [PubMed]
- Feudtner, C.; Kang, T.I.; Hexem, K.R.; Friedrichsdorf, S.J.; Osenga, K.; Siden, H.; Friebert, S.E.; Hays, R.M.; Dussel, V.; Wolfe, J. Pediatric Palliative Care Patients: A Prospective Multicenter Cohort Study. Pediatrics 2011, 127, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Fraser, L.K.; Lidstone, V.; Miller, M.; Aldridge, J.; Norman, P.; McKinney, P.A.; Parslow, R.C. Patterns of Diagnoses among Children and Young Adults with Life-Limiting Conditions: A Secondary Analysis of a National Dataset. Palliat. Med. 2014, 28, 513–520. [Google Scholar] [CrossRef]
- Wood, F.; Simpson, S.; Barnes, E.; Hain, R. Disease Trajectories and ACT/RCPCH Categories in Paediatric Palliative Care. Palliat. Med. 2010, 24, 796–806. [Google Scholar] [CrossRef]
- Garske, D.; Schmidt, P.; Hasan, C.; Wager, J.; Zernikow, B. Inpatient Paediatric Palliative Care– A Retrospective Study. Z. Für Palliat. 2016, 17, 302–307. [Google Scholar] [CrossRef]
- Allen, J.; Brenner, M.; Hauer, J.; Molloy, E.; McDonald, D. Severe Neurological Impairment: A Delphi Consensus-Based Definition. Eur. J. Paediatr. Neuro 2020. [Google Scholar] [CrossRef]
- Stallard, P.; Williams, L.; Lenton, S.; Velleman, R. Pain in Cognitively Impaired, Non-Communicating Children. Arch. Dis. Child. 2001, 85, 460–462. [Google Scholar] [CrossRef]
- Zernikow, B.; Michel, E.; Craig, F.; Anderson, B.J. Pediatric Palliative Care: Use of Opioids for the Management of Pain. Pediatr. Drugs 2009, 11, 129–151. [Google Scholar] [CrossRef]
- Hauer, J.M. Treating Dyspnea with Morphine Sulfate in Nonverbal Children with Neurological Impairment. Pediatr. Pulmonol. 2015, 50, E9–E12. [Google Scholar] [CrossRef]
- Hain, R.D.W.; Miser, A.; Devins, M.; Wallace, W.H.B. Strong Opioids in Pediatric Palliative Medicine. Pediatr. Drugs 2005, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.E.; Heathcote, L.C.; Anderson, B.; Grégoire, M.; Ljungman, G.; Eccleston, C. Non-steroidal Anti-inflammatory Drugs (NSAIDs) for Cancer-related Pain in Children and Adolescents. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef]
- Cooper, T.E.; Fisher, E.; Anderson, B.; Wilkinson, N.M.; Williams, D.G.; Eccleston, C. Paracetamol (Acetaminophen) for Chronic Non-cancer Pain in Children and Adolescents. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef]
- “Weak” Opioid Analgesics. Codeine, Dihydrocodeine and Tramadol: No Less Risky than Morphine. Prescr. Int. 2016, 25, 45–50. [Google Scholar]
- Bottos, S.; Chambers, C. The epidemiology of pain in developmental disabilities. In Pain in Children and Adults with Developmental Disabilities; Paul, H., Ed.; Brooks Publishing Co.: Baltimore, MD, USA, 2006; pp. 67–87. [Google Scholar]
- Breau, L.M.; Camfield, C.S.; McGrath, P.J.; Finley, G.A. The Incidence of Pain in Children With Severe Cognitive Impairments. Arch. Pediatr. Adol. Med. 2003, 157, 1219. [Google Scholar] [CrossRef]
- Niesters, M.; Overdyk, F.; Smith, T.; Aarts, L.; Dahan, A. Opioid-Induced Respiratory Depression in Paediatrics: A Review of Case Reports. Br. J. Anaesth. 2013, 110, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.; Simons, J.; Bray, L.; Arnott, J. Navigating Uncertainty: Health Professionals’ Knowledge, Skill, and Confidence in Assessing and Managing Pain in Children with Profound Cognitive Impairment. Pain Res. Manag. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Malviya, S.; Voepel-Lewis, T.; Tait, A.R.; Merkel, S.; Lauer, A.; Munro, H.; Farley, F. Pain Management in Children with and without Cognitive Impairment Following Spine Fusion Surgery. Pediatr. Anesth. 2001, 11, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.D. The Balance Between Effective Opioid-Based Pain Management and Patient Safety: Can It Be Achieved? J. Pediatr. Pharmacol. Ther. 2013, 18. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.E.; Fisher, E.; Gray, A.L.; Krane, E.; Sethna, N.; van Tilburg, M.A.; Zernikow, B.; Wiffen, P.J. Opioids for Chronic Non-cancer Pain in Children and Adolescents. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef]
- Dahan, A. Respiratory Depression with Opioids. J. Pain Palliat. Care Pharmacother. 2007, 21, 63–66. [Google Scholar] [CrossRef]
- YAMANAKA, T.; SADIKOT, R.T. Opioid Effect on Lungs. Respirology 2013, 18, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Pattinson, K.T.S. Opioids and the Control of Respiration. Br. J. Anaesth. 2008, 100, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467. [Google Scholar] [CrossRef] [PubMed]
- Voepel-Lewis, T.; Marinkovic, A.; Kostrzewa, A.; Tait, A.R.; Malviya, S. The Prevalence of and Risk Factors for Adverse Events in Children Receiving Patient-Controlled Analgesia by Proxy or Patient-Controlled Analgesia after Surgery. Anesth. Analg. 2008, 107, 70–75. [Google Scholar] [CrossRef]
- Jay, M.A.; Thomas, B.M.; Nandi, R.; Howard, R.F. Higher Risk of Opioid-Induced Respiratory Depression in Children with Neurodevelopmental Disability: A Retrospective Cohort Study of 12 904 Patients. Br. J. Anaesth. 2017, 118, 239–246. [Google Scholar] [CrossRef]
- Czarnecki, M.L.; Ferrise, A.S.; Mano, K.E.J.; Garwood, M.M.; Sharp, M.; Davies, H.; Weisman, S.J. Parent/Nurse-Controlled Analgesia for Children with Developmental Delay. Clin. J. Pain 2008, 24, 817–824. [Google Scholar] [CrossRef]
- Chidambaran, V.; Olbrecht, V.; Hossain, M.; Sadhasivam, S.; Rose, J.; Meyer, M.J. Risk Predictors of Opioid-Induced Critical Respiratory Events in Children: Naloxone Use as a Quality Measure of Opioid Safety. Pain Med. 2014, 15, 2139–2149. [Google Scholar] [CrossRef]
- Morton, N.S.; Errera, A. APA National Audit of Pediatric Opioid Infusions. Pediatric Anesth. 2010, 20, 119–125. [Google Scholar] [CrossRef]
- Monitto, C.L.; Greenberg, R.S.; Kost-Byerly, S.; Wetzel, R.; Billett, C.; Lebet, R.M.; Yaster, M. The Safety and Efficacy of Parent-/Nurse-Controlled Analgesia in Patients Less than Six Years of Age. Anesth. Analg. 2000, 91, 573–579. [Google Scholar] [CrossRef]
- Dreier, L.A.; Zernikow, B.; Wager, J. Quantifying the Language Barrier—A Total Survey of Parents’ Spoken Languages and Local Language Skills as Perceived by Different Professions in Pediatric Palliative Care. Children 2020, 7, 118. [Google Scholar] [CrossRef] [PubMed]
- The Association of Paediatric Palliative Medicine Master Formulary 5th Edition. Available online: https://www.appm.org.uk/_webedit/uploaded-files/All%20Files/Event%20Resources/2020%20APPM%20Master%20Formulary%202020%20protected.pdf (accessed on 26 September 2020).
- Likar, R. Transdermal Buprenorphine in the Management of Persistent Pain-Safety Aspects. Ther. Clin. Risk Manag. 2006, 2, 115–125. [Google Scholar] [PubMed]
- Feudtner, C.; Dai, D.; Hexem, K.R.; Luan, X.; Metjian, T.A. Prevalence of Polypharmacy Exposure Among Hospitalized Children in the United States. Arch. Pediatr. Adol. Med. 2012, 166, 9–16. [Google Scholar] [CrossRef]
- Fuentes, D.P.I.; Martin-Aragon, S.; Vendrell, M.C.-M. Medication Reconciliation upon Admission in Paediatric Hospital Setting: Preliminary Data. Int. J. Clin. Pharm.-Net 2020, 42, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Aston, J.; Huynh, C.; Sinclair, A.; Wilson, K.; Terry, D. Medication review of children on long term medications: A review of the literature. Arch. Dis. Child. 2016, 101, e2. [Google Scholar] [CrossRef] [PubMed]
- Wall, S.L.; Clarke, D.L.; Nauhaus, H.; Allorto, N.L. Barriers to Adequate Analgesia in Paediatric Burns Patients. S. Afr. Med. J. 2020, 110, 1032–1035. [Google Scholar] [CrossRef] [PubMed]
- Radbruch, L.; Glaeske, G.; Grond, S.; Münchberg, F.; Scherbaum, N.; Storz, E.; MA, K.T.; Zagermann-Muncke, P.; Zieglgänsberger, W.; Hoffmann-Menzel, H.; et al. Topical Review on the Abuse and Misuse Potential of Tramadol and Tilidine in Germany. Subst. Abus. 2013, 34, 313–320. [Google Scholar] [CrossRef]
- Grün, B.; Merkel, U.; Riedel, K.; Weiss, J.; Mikus, G. Contribution of CYP2C19 and CYP3A4 to the Formation of the Active Nortilidine from the Prodrug Tilidine. Br. J. Clin. Pharmacol. 2012, 74, 854–863. [Google Scholar] [CrossRef]
- Dahan, A.; Yassen, A.; Bijl, H.; Romberg, R.; Sarton, E.; Teppema, L.; Olofsen, E.; Danhof, M. Comparison of the Respiratory Effects of Intravenous Buprenorphine and Fentanyl in Humans and Rats. BJA Br. J. Anaesth. 2005, 94, 825–834. [Google Scholar] [CrossRef]
- Brown, K.A.; Laferrière, A.; Lakheeram, I.; Moss, I.R. Recurrent Hypoxemia in Children Is Associated with Increased Analgesic Sensitivity to Opiates. Anesthesiology 2006, 105, 665–669. [Google Scholar] [CrossRef]
- Hunt, A.; Goldman, A.; Seers, K.; Crichton, N.; Mastroyannopoulou, K.; Moffat, V.; Oulton, K.; Brady, M. Clinical Validation of the Paediatric Pain Profile. Dev. Med. Child. Neurol. 2003, 46. [Google Scholar] [CrossRef]
- Malviya, S.; Voepel-Lewis, T.; Burke, C.; Merkel, S.; Tait, A.R. The Revised FLACC Observational Pain Tool: Improved Reliability and Validity for Pain Assessment in Children with Cognitive Impairment. Pediatr. Anesth. 2006, 16, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Niesters, M.; Mahajan, R.P.; Aarts, L.; Dahan, A. High-Inspired Oxygen Concentration Further Impairs Opioid-Induced Respiratory Depression. BJA Br. J. Anaesth. 2013, 110, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Lightdale, J.R.; Goldmann, D.A.; Feldman, H.A.; Newburg, A.R.; DiNardo, J.A.; Fox, V.L. Microstream Capnography Improves Patient Monitoring During Moderate Sedation: A Randomized, Controlled Trial. Pediatrics 2006, 117, e1170–e1178. [Google Scholar] [CrossRef]
- Krauss, B.; Hess, D.R. Capnography for Procedural Sedation and Analgesia in the Emergency Department. Ann. Emerg. Med. 2007, 50, 172–181. [Google Scholar] [CrossRef]
- Deitch, K.; Chudnofsky, C.R.; Dominici, P. The Utility of Supplemental Oxygen During Emergency Department Procedural Sedation and Analgesia with Midazolam and Fentanyl: A Randomized, Controlled Trial. Ann. Emerg. Med. 2007, 49, 1–8. [Google Scholar] [CrossRef]
- Ribbers, S.; Wager, J.; Hartenstein-Pinter, A.; Zernikow, B.; Reuther, M. Core Outcome Domains of Pediatric Palliative Care for Children with Severe Neurological Impairment and Their Families: A Qualitative Interview Study. Palliat. Med. 2019, 269216319885818. [Google Scholar] [CrossRef]
- Sullivan, P.; Lambert, B.; Rose, M.; Ford-Adams, M.; Johnson, A.; Griffiths, P. Prevalence and Severity of Feeding and Nutritional Problems in Children with Neurological Impairment: Oxford Feeding Study. Dev. Med. Child. Neurol. 2000, 42, 674–680. [Google Scholar] [CrossRef]
- Olkkola, K.T.; Hamunen, K.; Maunuksela, E.-L. Clinical Pharmacokinetics and Pharmacodynamics of Opioid Analgesics in Infants and Children. Clin. Pharm. 1995, 28, 385–404. [Google Scholar] [CrossRef]
- Kuip, E.J.M.; Zandvliet, M.L.; Koolen, S.L.W.; Mathijssen, R.H.J.; Rijt, C.C.D. A Review of Factors Explaining Variability in Fentanyl Pharmacokinetics; Focus on Implications for Cancer Patients. Br. J. Clin. Pharmacol. 2017, 83, 294–313. [Google Scholar] [CrossRef]
- Hauer, J.; Houtrow, A.J. Pain Assessment and Treatment in Children with Significant Impairment of the Central Nervous System. Pediatrics 2017, 139, e20171002. [Google Scholar] [CrossRef] [PubMed]
- Frader, J.; Derrington, S.; Morgan, E. Don’t Forget Palliative Patients. JAMA Pediatr. 2015, 169, 285. [Google Scholar] [CrossRef] [PubMed]
- OBERLANDER, T.F.; O’DONNELL, M.E.; MONTGOMERY, C.J. Pain in Children with Significant Neurological Impairment. J. Dev. Behav. Pediatr. 1999, 20, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Sisk, B.A.; Feudtner, C.; Bluebond-Langner, M.; Sourkes, B.; Hinds, P.S.; Wolfe, J. Response to Suffering of the Seriously Ill Child: A History of Palliative Care for Children. Pediatrics 2019, 145, e20191741. [Google Scholar] [CrossRef]
| opioid [MeSH Terms] OR opioid OR morphine [MeSH Terms] OR morphine OR morphine derivatives [MeSH Terms] OR Fentanyl [MeSH Terms] OR Fentanyl OR Buprenorphine [MeSH Terms] OR Buprenorphine OR Methadone [MeSH Terms] OR Methadone OR Levomethadone OR Tramadol [MeSH Terms] OR Tramadol OR Tilidine [MeSH Terms] OR Tilidine | AND | Apnea [MeSH Terms] OR apnea OR “respiratory depression” | AND | neuromuscular diseases [MeSH Terms] OR neuromuscular diseases OR neurodegenerative diseases [MeSH Terms] OR neurodegenerative diseases OR neurotoxicity Syndromes [MeSH Terms] OR neurotoxicity Syndromes OR neurological manifestations [MeSH Terms] OR neurological manifestations OR “psychomotor impairment” OR “severe psychomotor impairment” OR “developmental disabilities” | AND | child [MeSH Terms] OR child * OR Adolescent [MeSH Terms] OR Adolescent * OR infant [MeSH Terms] OR Infant * OR pediatrics [MeSH Terms] OR Pediatric * |
| Reference | Study Design | Objective | Sample Description, Size, Age | Definition of Critical Respiratory Events | Risk Factors and Main Results |
|---|---|---|---|---|---|
| Voepel-Lewis (2008) [26] | Retrospective chart review | Comparing the prevalence of clinically significant adverse events in children receiving Patient-Controlled Analgesia (PCA) vs. Patient-Controlled Analgesia by Proxy (PCAP) after surgery. | Random sample of children receiving PCA and PCAP. n = 157 PCA age (years) 13.1 ± 3.2 n = 145 PCAP age (years) 7.08 ± 5.3 | Respiratory depression requiring rescue events: Treated with naloxone, airway management, or escalation of care | Opioid dose and cognitive impairment were independent predictors of rescue events irrespective of patients receiving PCA or PCAP. Despite reduced opioid consumption, the odds ratio for a rescue event in patients with cognitive impairment was 2.4 (CI 1.3–4.2). Additional risk factors associated included orthopedic surgery, respiratory comorbidity, continuous basal opioid infusion, diazepam use, and higher opioid doses. |
| Jay (2017) [27] | Retrospective cohort study | Quantification of the risks and effectiveness of Nurse-Controlled Analgesia (NCA) for postoperative pain in children with neurodevelopmental disabilities compared to a control group. | Patients who received NCA and were identified from the clinical patient record as having neurodevelopmental disabilities were divided into a neuro-developmental disabilities group (NDG) and a control group (CG). n = 12904 age distribution not reported | Respiratory depression defined as a depression of the respiratory rate below an age-defined rate | The cumulative incidence of OIRD in the neurodevelopmental disability group was 1.09% vs. 0.59% in the control group [odds ratio 1.8 (98% chance that the true odds ratio was >1)]. Significant interactions between postoperative morphine dose and SNI were shown in a logistic regression model. Children with cerebral palsy, Down’s syndrome, and encephalopathy were at the highest risk of developing respiratory depression in the neurodevelopmental disability group. Children with SNI were suspected of having an increased risk of respiratory depression because of an increased incidence of impaired respiratory drive, cardiorespiratory deficits, neuromuscular and postural abnormalities, and gastroesophageal reflux. |
| Czarnecki (2008) [28] | Retrospective chart review | Evaluation of outcomes associated with mainly postoperative Parent/Nurse- controlled Analgesia (PNCA) in pediatric patients with identified developmental delay. | Patients who received PNCA and were identified as being developmentally delayed based on clinical documentation. n = 71 age (years) 9.9 ± 5.28 | Requirement of naloxone for sedation or respiratory depression. | 2.8% of the patients received naloxone to treat side effects of opioids (oversedation or respiratory depression). Adjuvant sedating medications (diazepam, droperidol, chloral hydrate, and diphenhydramine) may have contributed to respiratory depression. |
| Chidambaran (2014) [29] | Retrospective chart review | Naloxone usage for opioid-induced critical respiratory events in children as a quality measure of opioid safety in patients receiving postoperative and other opioid therapy. | All patients who received naloxone for opioid-induced critical respiratory events. n = 38 age (years) 8.7 ± 8.0 | Requirement of naloxone for respiratory depression. | Age <1 year, underweight, obesity, history of prematurity syndrome, developmental delay, obstructive sleep apnea, and respiratory, hepatic, and neurological comorbidities were significant risk factors for early respiratory depression associated with opioid treatment. The unadjusted odds ratios for the need for administration of naloxone were 3.24 (CI 1.36–7.47) for the presence of a syndrome, 4.99 (CI 2.17–11.15) for developmental delay, and 3.87 (CI 1.83–8.07) for neurological impairment. |
| Morton (2010) [30] | Prospective cohort study | Determination of the incidence, nature, and severity of serious clinical incidents associated with continuous opioid infusion, Patient-Controlled Analgesia (PCA), and Nurse-Controlled Analgesia (NCA) in pediatric patients. | Sample of all pediatric patients who received opioid-infusion, PCA, and NCA. n = 10726 age < 1 month = 344 age 1 month–1 year = 1383 age 1–8 years= 3433 age 8–18 years = 5566 | Death or permanent harm/ harm but full recovery leading to discontinuation of the technique or requiring significant intervention/ potential but no actual harm. | Eight of the fourteen reports of respiratory depression received naloxone; they were all very young or had significant neurodevelopmental, respiratory, or cardiac comorbidities. Avoidance of concurrent sedatives or opioids and awareness of comorbidities can improve patient safety. |
| Monitto et al. (2000) [31] | Prospective cohort study | Determination of patient demographics, analgesia effectiveness, and the incidence of complications in pediatric patients receiving Parent/Nurse- Controlled Analgesia (PNCA). | All patients <6 years of age who received PNCA. n = 212 age (years) 2.3 ± 1.7 | Apnea or oxygen desaturation | No specific risk factor was associated with naloxone administration in nonsurgical and postoperative children under six years of age. Patients’ clinical characteristics were found to be predisposing factors for excessive sedation or respiratory compromise. In this context, additional sedatives, development delay, and congenital anomalies were named. |
| Case 1 | |
| Underlying disease and main symptoms | Hypoxic-ischemic encephalopathy Preterm birth at 26 weeks Cardiopulmonary resuscitation in severe sepsis at age 16 Spastic cerebral palsy (GMFCS level V) Contractures Thoracic scoliosis Dislocated hip dysplasia Symptomatic epilepsy with epileptic spasms Hypothyroidism Underweight |
| Age [years] | 18.8 |
| Weight [kg] | 33.2 |
| Length [cm] | 140 |
| BMI [kg/m²] (Percentile) | 16.8 (2.) |
| Indication for opioid therapy | Musculoskeletal pain |
| Opioid medication (Route of administration) | Morphine (Enteral) |
| Dosage | 4 × 2 mg |
| Daily oral morphine equivalent dose [mg/kg/d] | 0.241 |
| Relevant comedication during OIRD | Levetiracetam, valproate, dronabinol, baclofen, chloral hydrate, melatonin, ibuprofen |
| Opioid-induced respiratory event | Hypopnea (lowest respiratory rate 7/min) Oxygen desaturation (lowest oxygen saturation 82%) |
| OIRD at day of opioid treatment | 2 |
| Interventions | Repeated stimulation Oxygen supply Termination of opioid treatment Opioid switch to levomethadone |
| Case 2 | |
| Underlying disease and main symptoms | Early infantile epileptic encephalopathy (Ohtahara syndrome) Obstructive sleep apnea Spastic cerebral palsy (GMFCS level V) Contractures Respiratory failure type I |
| Age [years] | 15 |
| Weight [kg] | 23.5 |
| Length [cm] | 105 |
| BMI [kg/m²] (Percentile) | 21.3 (68) |
| Indication for opioid therapy | Severe neuroirritability with pain-like behavior |
| Opioid medication (Route of administration) | Levomethadone (Enteral) |
| Dosage | 3 × 1 mg |
| Daily oral morphine equivalent dose [mg/kg/d] | 1.02 [33] |
| Relevant comedication during OIRD | Lamotrigine, risperidone, domperidone, melatonin |
| Opioid-induced respiratory event | Apnea Oxygen desaturation (lowest oxygen saturation 88%) |
| OIRD at day of opioid treatment | 8 |
| Interventions | Repeated stimulation Oxygen supply Initial termination of opioid treatment |
| Case 3 | |
| Underlying disease and main symptoms | Lissencephaly Ventriculoatrial shunt Focal seizures Spastic cerebral palsy (GMFCS level V) Contractures Thoracic scoliosis Hip dysplasia Hypothyroidism |
| Age [years] | 20.6 |
| Weight [kg] | 38.9 |
| Length [cm] | 170 |
| BMI [kg/m²] (Percentile) | 13.8 (<1) |
| Indication for opioid therapy | Musculoskeletal pain |
| Opioid medication (Route of administration) | Buprenorphine (Transdermal) |
| Dosage | 5 µg/h |
| Daily oral morphine equivalent dose [mg/kg/d] | 0.308 [33] |
| Relevant comedication during OIRD | Oxcarbazepine, valproate, metamizole |
| Opioid-induced respiratory event | Repeated oxygen desaturation (lowest oxygen saturation 58%) |
| OIRD at day of opioid treatment | 1 |
| Interventions | Repeated stimulation Oxygen supply Initial termination of opioid treatment Dose reduction |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauritz, M.D.; Hasan, C.; Dreier, L.A.; Schmidt, P.; Zernikow, B. Opioid-Induced Respiratory Depression in Pediatric Palliative Care Patients with Severe Neurological Impairment—A Scoping Literature Review and Case Reports. Children 2020, 7, 312. https://doi.org/10.3390/children7120312
Mauritz MD, Hasan C, Dreier LA, Schmidt P, Zernikow B. Opioid-Induced Respiratory Depression in Pediatric Palliative Care Patients with Severe Neurological Impairment—A Scoping Literature Review and Case Reports. Children. 2020; 7(12):312. https://doi.org/10.3390/children7120312
Chicago/Turabian StyleMauritz, Maximilian David, Carola Hasan, Larissa Alice Dreier, Pia Schmidt, and Boris Zernikow. 2020. "Opioid-Induced Respiratory Depression in Pediatric Palliative Care Patients with Severe Neurological Impairment—A Scoping Literature Review and Case Reports" Children 7, no. 12: 312. https://doi.org/10.3390/children7120312
APA StyleMauritz, M. D., Hasan, C., Dreier, L. A., Schmidt, P., & Zernikow, B. (2020). Opioid-Induced Respiratory Depression in Pediatric Palliative Care Patients with Severe Neurological Impairment—A Scoping Literature Review and Case Reports. Children, 7(12), 312. https://doi.org/10.3390/children7120312







