Figure 1.
The relationship between pulmonary to systemic flow ratio (Qp/Qs) and stretched diameter of the atrial septal defect is plotted. Regression line (solid line) along with 95% confidence lines (interrupted lines) is shown. Note significant (
p < 0.05) correlation with an r value of 0.55. Reproduced from Rao P.S., et al. [
2].
Figure 1.
The relationship between pulmonary to systemic flow ratio (Qp/Qs) and stretched diameter of the atrial septal defect is plotted. Regression line (solid line) along with 95% confidence lines (interrupted lines) is shown. Note significant (
p < 0.05) correlation with an r value of 0.55. Reproduced from Rao P.S., et al. [
2].
Figure 2.
Plot similar to
Figure 1 showing the relationship of angiographic diameter of the atrial septal defect with stretched diameter of the atrial septal defect: Note significant (
p < 0.05) correlation with an r value of 0.55. Reproduced from Rao P.S., et al. [
2].
Figure 2.
Plot similar to
Figure 1 showing the relationship of angiographic diameter of the atrial septal defect with stretched diameter of the atrial septal defect: Note significant (
p < 0.05) correlation with an r value of 0.55. Reproduced from Rao P.S., et al. [
2].
Figure 3.
Plot similar to
Figure 1 and
Figure 2 showing the relationship of transthoracic echocardiographic diameter of the atrial septal defect with stretched diameter of the atrial septal defect: Note significant (
p < 0.0002) correlation with an r value of 0.82. Reproduced from Rao P.S., et al. [
2].
Figure 3.
Plot similar to
Figure 1 and
Figure 2 showing the relationship of transthoracic echocardiographic diameter of the atrial septal defect with stretched diameter of the atrial septal defect: Note significant (
p < 0.0002) correlation with an r value of 0.82. Reproduced from Rao P.S., et al. [
2].
Figure 4.
Plot similar to
Figure 1,
Figure 2 and
Figure 3 showing the relationship of predicted atrial septal defect (ASD) diameter by formula (1.05 × echo size in millimeters) + 5.49, with measured stretched size of the atrial septal defect: Note significant (
p < 0.001) correlation with an r value of 0.9. Reproduced from Rao P.S., et al. [
3].
Figure 4.
Plot similar to
Figure 1,
Figure 2 and
Figure 3 showing the relationship of predicted atrial septal defect (ASD) diameter by formula (1.05 × echo size in millimeters) + 5.49, with measured stretched size of the atrial septal defect: Note significant (
p < 0.001) correlation with an r value of 0.9. Reproduced from Rao P.S., et al. [
3].
Figure 5.
Drawings of 2D echo images of the atrial septum in subcostal long-axis (
A), subcostal short-axis (
B), and apical four-chamber (
C) views demonstrating how measurement of the atrial septal defect (ASD), length of the atrial septum (LAS), superior rim (SR), and inferior rim (IR) are obtained. LA, left atrium; LV, left ventricle; RA, right atrium; RPA, right pulmonary artery; RV, right ventricle; SVC, superior vena cava. Reproduced from Reddy S.C.B., et al. [
7].
Figure 5.
Drawings of 2D echo images of the atrial septum in subcostal long-axis (
A), subcostal short-axis (
B), and apical four-chamber (
C) views demonstrating how measurement of the atrial septal defect (ASD), length of the atrial septum (LAS), superior rim (SR), and inferior rim (IR) are obtained. LA, left atrium; LV, left ventricle; RA, right atrium; RPA, right pulmonary artery; RV, right ventricle; SVC, superior vena cava. Reproduced from Reddy S.C.B., et al. [
7].
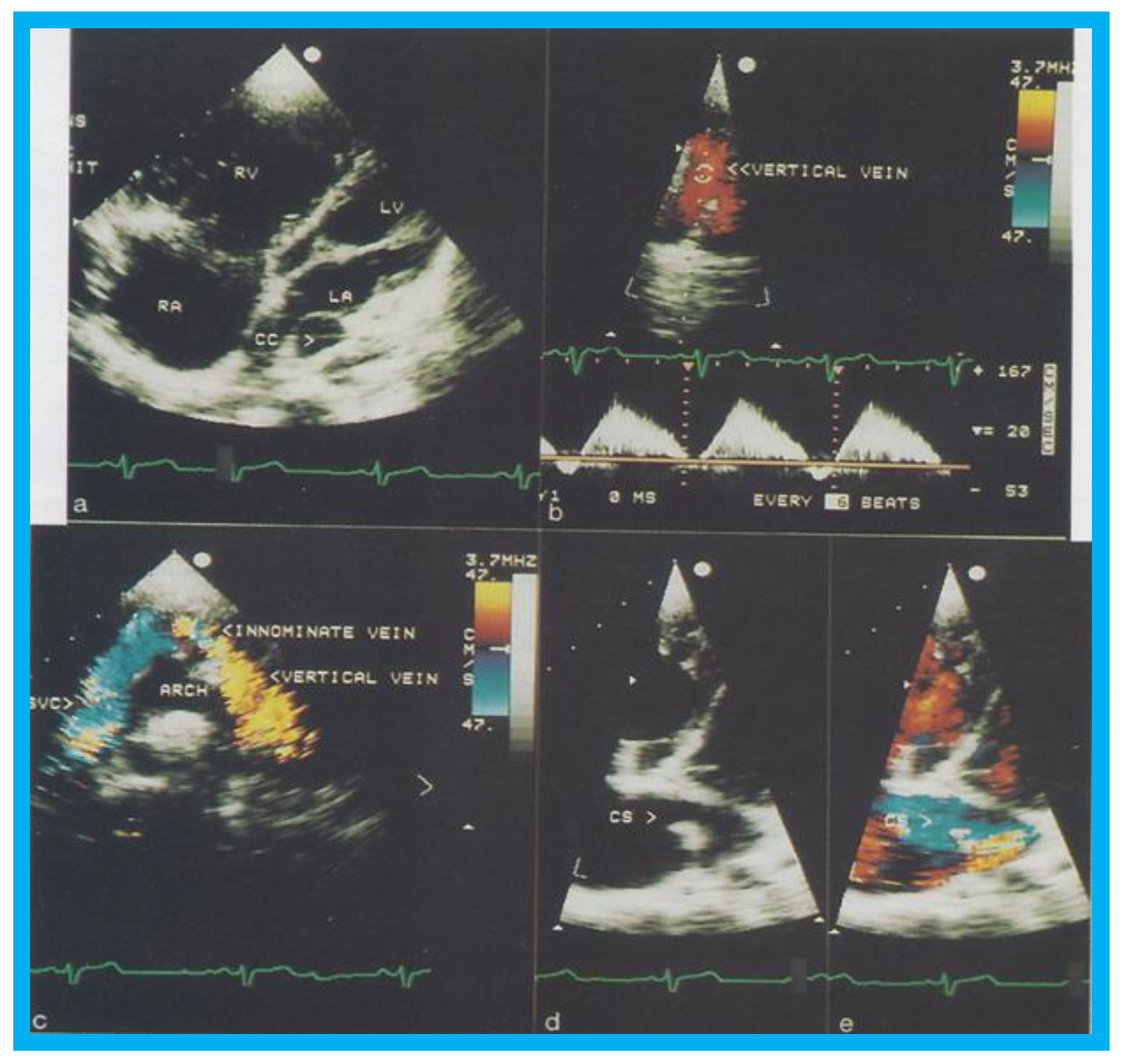
Figure 6.
Selected two-dimensional (
a) and color flow images (
b–
e) of a patient (case 1) with mixed type of total anomalous pulmonary venous connection are shown. In (
a), dilated right atrium (RA) and right ventricle (RV) and common pulmonary venous confluence (CC) are illustrated. In (
b,
c), spectral and color flow images demonstrate the vertical vein draining into the innominate vein and superior vena cava (SVC). In (
d,
e), dilated coronary sinus (CS) with mosaic color flow pattern of pulmonary venous entry are apparent. ARCH, aortic arch; LA, left atrium; LV, left ventricle. Reproduced from Reddy S.C.B., et al. [
8].
Figure 6.
Selected two-dimensional (
a) and color flow images (
b–
e) of a patient (case 1) with mixed type of total anomalous pulmonary venous connection are shown. In (
a), dilated right atrium (RA) and right ventricle (RV) and common pulmonary venous confluence (CC) are illustrated. In (
b,
c), spectral and color flow images demonstrate the vertical vein draining into the innominate vein and superior vena cava (SVC). In (
d,
e), dilated coronary sinus (CS) with mosaic color flow pattern of pulmonary venous entry are apparent. ARCH, aortic arch; LA, left atrium; LV, left ventricle. Reproduced from Reddy S.C.B., et al. [
8].
Figure 7.
Selected cineangiographic frames from postero-anterior (
A,
D,
E) and lateral (
B,
C) views of case 1 (
A–
C) and case 2 (
D,
E) demonstrating mixed total anomalous pulmonary venous connection. In (
A,
B), levo-angiographic frames following right pulmonary artery cineangiogram demonstrated entry of right pulmonary vein (RPV) into the coronary sinus (CS) in case 1. In (
C) is the selected angiographic frame from left pulmonary vein (LPV) cineangiogram demonstrating pulmonary venous drainage into the vertical vein (V) and then into the innominate vein (INN) and superior vena cava (SVC), also of case 1. In (
D), levo-angiographic frame following right pulmonary artery cineangiogram demonstrating entry of right pulmonary vein (RPV) into the coronary sinus (CS) in case 2 is shown. In (
E), levo-angiographic frame following left pulmonary artery cineangiogram demonstrating drainage of left pulmonary veins (LPV) into the vertical vein (V) and then into the INN and SVC of case 2 is shown. In (
A,
B,
D), the connections of the RPVs to the CS are marked with arrows to improve clarity. Reproduced from Reddy S.C.B., et al. [
9].
Figure 7.
Selected cineangiographic frames from postero-anterior (
A,
D,
E) and lateral (
B,
C) views of case 1 (
A–
C) and case 2 (
D,
E) demonstrating mixed total anomalous pulmonary venous connection. In (
A,
B), levo-angiographic frames following right pulmonary artery cineangiogram demonstrated entry of right pulmonary vein (RPV) into the coronary sinus (CS) in case 1. In (
C) is the selected angiographic frame from left pulmonary vein (LPV) cineangiogram demonstrating pulmonary venous drainage into the vertical vein (V) and then into the innominate vein (INN) and superior vena cava (SVC), also of case 1. In (
D), levo-angiographic frame following right pulmonary artery cineangiogram demonstrating entry of right pulmonary vein (RPV) into the coronary sinus (CS) in case 2 is shown. In (
E), levo-angiographic frame following left pulmonary artery cineangiogram demonstrating drainage of left pulmonary veins (LPV) into the vertical vein (V) and then into the INN and SVC of case 2 is shown. In (
A,
B,
D), the connections of the RPVs to the CS are marked with arrows to improve clarity. Reproduced from Reddy S.C.B., et al. [
9].
![Children 07 00034 g007]()
Figure 8.
Bar graph illustrating the effect of transcatheter closure of atrial septal defect (ASD) with buttoned device on the left and right ventricular end-diastolic dimensions: Left panel shows that the dimension of the right ventricle fell (
p < 0.01) immediately following ASD closure. There was an additional but statistically insignificant (
p > 0.1) decrease in right ventricular dimension at follow-up (FU). The right panel demonstrates that there is no statistically significant change (
p > 05) either immediately after ASD closure or at FU. POST (on the day following ASD closure), on the day following ASD closure; PRE (prior to ASD closure), prior to ASD closure. Reproduced from Rao P.S., et al. [
9].
Figure 8.
Bar graph illustrating the effect of transcatheter closure of atrial septal defect (ASD) with buttoned device on the left and right ventricular end-diastolic dimensions: Left panel shows that the dimension of the right ventricle fell (
p < 0.01) immediately following ASD closure. There was an additional but statistically insignificant (
p > 0.1) decrease in right ventricular dimension at follow-up (FU). The right panel demonstrates that there is no statistically significant change (
p > 05) either immediately after ASD closure or at FU. POST (on the day following ASD closure), on the day following ASD closure; PRE (prior to ASD closure), prior to ASD closure. Reproduced from Rao P.S., et al. [
9].
Figure 9.
Graph illustrating the effect of transcatheter closure of atrial septal defect (ASD) with buttoned device on the ventricular septal motion: Prior to ASD closure (PRE), the ventricular septal motion is either paradoxical or flat in the majority of patients. Immediately after ASD occlusion (POST), the ventricular septal motion is normal in all but one patient, and at follow-up (FU), the ventricular septal motion returned to normal in all patients. Reproduced from Rao P.S., et al. [
9].
Figure 9.
Graph illustrating the effect of transcatheter closure of atrial septal defect (ASD) with buttoned device on the ventricular septal motion: Prior to ASD closure (PRE), the ventricular septal motion is either paradoxical or flat in the majority of patients. Immediately after ASD occlusion (POST), the ventricular septal motion is normal in all but one patient, and at follow-up (FU), the ventricular septal motion returned to normal in all patients. Reproduced from Rao P.S., et al. [
9].
Figure 10.
Selected video frames from 2D echo studies in subcostal position of the atrial septum in long-axis view prior to (
a); immediately after (
b); and at one (
c), six (
d), 12 (
e), and 24 (
f) months following atrial septal defect (ASD) closure illustrating the results of transcatheter closure of ASD with buttoned device. Note the position of the device (D) across the ASD (
b–
f) during follow-up, the device appears to be incorporated into the atrial septum. On pulsed and color-Doppler studies concurrent with two-dimensional echo studies, there was no evidence for left over shunt (not shown). LA, left atrium; RA, right atrium. Reproduced from Rao P.S., et al. [
9].
Figure 10.
Selected video frames from 2D echo studies in subcostal position of the atrial septum in long-axis view prior to (
a); immediately after (
b); and at one (
c), six (
d), 12 (
e), and 24 (
f) months following atrial septal defect (ASD) closure illustrating the results of transcatheter closure of ASD with buttoned device. Note the position of the device (D) across the ASD (
b–
f) during follow-up, the device appears to be incorporated into the atrial septum. On pulsed and color-Doppler studies concurrent with two-dimensional echo studies, there was no evidence for left over shunt (not shown). LA, left atrium; RA, right atrium. Reproduced from Rao P.S., et al. [
9].
Figure 11.
Selected video frames from transesophageal echocardiograms in an adult with presumed paradoxical embolism prior to (Pre) (
a–
c) and six months (post) (
d) after closure of patent foramen ovale with buttoned device: Short axis views illustrate aorta (Ao) in the middle and right atrium (RA) and left atrium (LA) to the right before (
a) and after (
b) injection of agitated saline into the RA. Note that there are no contrast bubbles in (
b). However, with contrast and Valsalva (Val) (
c), the LA is opacified (C filled arrow). Study similar to c, performed six months following (
d) (post, Cont, and Val) shows the device (unfilled arrow D) without contrast bubbles in LA. Note that contrast bubbles are seen in RA in (
b–
d). Reproduced from Rao P.S., et al. [
9].
Figure 11.
Selected video frames from transesophageal echocardiograms in an adult with presumed paradoxical embolism prior to (Pre) (
a–
c) and six months (post) (
d) after closure of patent foramen ovale with buttoned device: Short axis views illustrate aorta (Ao) in the middle and right atrium (RA) and left atrium (LA) to the right before (
a) and after (
b) injection of agitated saline into the RA. Note that there are no contrast bubbles in (
b). However, with contrast and Valsalva (Val) (
c), the LA is opacified (C filled arrow). Study similar to c, performed six months following (
d) (post, Cont, and Val) shows the device (unfilled arrow D) without contrast bubbles in LA. Note that contrast bubbles are seen in RA in (
b–
d). Reproduced from Rao P.S., et al. [
9].
Figure 12.
Selected video frames from parasternal long-axis view in a normal child (
A) and in a child with aberrant coronary artery (
B): Note a cross-sectional image of the coronary artery (CA) is seen in (
B) in the anterior wall of the aorta (AO) while such is not seen in (
A). LA, left atrium; LV, left ventricle; RV, right ventricle. Reproduced from Jureidini S.B., et al. [
15].
Figure 12.
Selected video frames from parasternal long-axis view in a normal child (
A) and in a child with aberrant coronary artery (
B): Note a cross-sectional image of the coronary artery (CA) is seen in (
B) in the anterior wall of the aorta (AO) while such is not seen in (
A). LA, left atrium; LV, left ventricle; RV, right ventricle. Reproduced from Jureidini S.B., et al. [
15].
Figure 13.
Graph showing calculated Doppler peak instantaneous gradients prior to (Pre) and one day after (Post) balloon pulmonary valvuloplasty (BPV) and at intermediate-term (ITFU) and long-term (LTFU) follow-up: Significant decrease (
p < 0.001) following BPV (Pre vs. Post) occurred which did not change (
p > 0.1) at ITFU. But, at LTFU, there was a further decrease (
p < 0.001) in the Doppler-calculated gradients. Mean + standard deviation (SD) are depicted. Reproduced from Rao P.S., et al. [
16].
Figure 13.
Graph showing calculated Doppler peak instantaneous gradients prior to (Pre) and one day after (Post) balloon pulmonary valvuloplasty (BPV) and at intermediate-term (ITFU) and long-term (LTFU) follow-up: Significant decrease (
p < 0.001) following BPV (Pre vs. Post) occurred which did not change (
p > 0.1) at ITFU. But, at LTFU, there was a further decrease (
p < 0.001) in the Doppler-calculated gradients. Mean + standard deviation (SD) are depicted. Reproduced from Rao P.S., et al. [
16].
Figure 14.
Graph showing end diastolic dimension of the right ventricle (RV) prior to (Pre), one day following (Post), at intermediate-term (ITFU), and at long-term (LTFU) follow-up after balloon pulmonary valvuloplasty (BPV). A significant reduction (
p < 0.05) in RV size immediately after BPV was seen. No further change at ITFU and LTFU occurred. Increased (
p < 0.05) prevalence of flat septal motion was observed at LTFU (see
Figure 16). None of the patients had paradoxical septal motion. Mean + standard deviation (SD) are depicted. Reproduced from Rao P.S., et al. [
16].
Figure 14.
Graph showing end diastolic dimension of the right ventricle (RV) prior to (Pre), one day following (Post), at intermediate-term (ITFU), and at long-term (LTFU) follow-up after balloon pulmonary valvuloplasty (BPV). A significant reduction (
p < 0.05) in RV size immediately after BPV was seen. No further change at ITFU and LTFU occurred. Increased (
p < 0.05) prevalence of flat septal motion was observed at LTFU (see
Figure 16). None of the patients had paradoxical septal motion. Mean + standard deviation (SD) are depicted. Reproduced from Rao P.S., et al. [
16].
Figure 15.
Bar graph showing Doppler-graded pulmonary insufficiency prior to (Pre) and one day after (Post) balloon pulmonary valvuloplasty and at intermediate-term (ITFU) and long-term (LTFU) follow-up: A gradual but significant increase (
p < 0.05 to
p < 0.001) in the incidence of pulmonary insufficiency is seen. Reproduced from Rao P.S., et al. [
16].
Figure 15.
Bar graph showing Doppler-graded pulmonary insufficiency prior to (Pre) and one day after (Post) balloon pulmonary valvuloplasty and at intermediate-term (ITFU) and long-term (LTFU) follow-up: A gradual but significant increase (
p < 0.05 to
p < 0.001) in the incidence of pulmonary insufficiency is seen. Reproduced from Rao P.S., et al. [
16].
Figure 16.
Bar graph showing the prevalence of inter-ventricular septal motion prior to (Pre) and one day after (Post) balloon pulmonary valvuloplasty and at intermediate-term (ITFU) and long-term (LTFU) follow-up: Note significant increase (
p < 0.05) in the incidence of flat septal motion at LTFU. No patient was observed to have paradoxical septal motion. Reproduced from Rao P.S., et al. [
16].
Figure 16.
Bar graph showing the prevalence of inter-ventricular septal motion prior to (Pre) and one day after (Post) balloon pulmonary valvuloplasty and at intermediate-term (ITFU) and long-term (LTFU) follow-up: Note significant increase (
p < 0.05) in the incidence of flat septal motion at LTFU. No patient was observed to have paradoxical septal motion. Reproduced from Rao P.S., et al. [
16].
Figure 17.
(
A) Selected video frame from apical four chamber projection indicating reversal of the ventricles: The attachment of the mitral valve (MV) leaflet is higher than that of the tricuspid valve (TV), suggesting that the morphologic right ventricle (RV) is on the left side and that the morphologic left ventricle (LV) is on the right side. The right atrium (RA) drains into morphologic LV, and the left atrium (LA) is connected to the morphologic RV. Also note that the medial leaflet of the TV is plastered on to the ventricular septum suggesting Ebstein’s type of morphologic TV. (
B,
C) Selected video frames from parasternal long axis view demonstrate the aneurysm (Aneu) with color-Doppler turbulence (
C). (
D) This frame shows a high peak Doppler flow (3.5 m/s), which indicates a peak instantaneous gradient of 49 mmHg. Reproduced from Reddy S.C.B., et al. [
22].
Figure 17.
(
A) Selected video frame from apical four chamber projection indicating reversal of the ventricles: The attachment of the mitral valve (MV) leaflet is higher than that of the tricuspid valve (TV), suggesting that the morphologic right ventricle (RV) is on the left side and that the morphologic left ventricle (LV) is on the right side. The right atrium (RA) drains into morphologic LV, and the left atrium (LA) is connected to the morphologic RV. Also note that the medial leaflet of the TV is plastered on to the ventricular septum suggesting Ebstein’s type of morphologic TV. (
B,
C) Selected video frames from parasternal long axis view demonstrate the aneurysm (Aneu) with color-Doppler turbulence (
C). (
D) This frame shows a high peak Doppler flow (3.5 m/s), which indicates a peak instantaneous gradient of 49 mmHg. Reproduced from Reddy S.C.B., et al. [
22].
Figure 18.
Selected video frames by two-dimensional (
A) and with color flow imaging (
B) from apical four-chamber view from the right chest demonstrating morphologic right (MRV) and morphologic left (MLV) ventricles and a large VSD (ventricular septal defect) in a patient with dextrocardia. Reproduced from Yarrabolu T.R., et al. [
23].
Figure 18.
Selected video frames by two-dimensional (
A) and with color flow imaging (
B) from apical four-chamber view from the right chest demonstrating morphologic right (MRV) and morphologic left (MLV) ventricles and a large VSD (ventricular septal defect) in a patient with dextrocardia. Reproduced from Yarrabolu T.R., et al. [
23].
Figure 19.
Selected video frames from a right parasternal (
A) and subcostal four chamber (
B) views of the morphologic left ventricle (MLV) demonstrating aneurysm (Anu) projecting into the MLV outflow tract, producing obstruction: The Anu is located just below the pulmonary valve (PV). The main pulmonary artery (MPA) is dilated. (
C) An enlarged view of B illustrating the aneurysm and MPA dilatation. Reproduced from Yarrabolu T.R., et al. [
23].
Figure 19.
Selected video frames from a right parasternal (
A) and subcostal four chamber (
B) views of the morphologic left ventricle (MLV) demonstrating aneurysm (Anu) projecting into the MLV outflow tract, producing obstruction: The Anu is located just below the pulmonary valve (PV). The main pulmonary artery (MPA) is dilated. (
C) An enlarged view of B illustrating the aneurysm and MPA dilatation. Reproduced from Yarrabolu T.R., et al. [
23].
Figure 20.
(
A) Selected video frame from a subcostal four chamber view (similar to
Figure 19B) with color-Doppler demonstrating turbulent flow (TF) in the pulmonary outflow tract. (
B) Continuous wave Doppler recording across the pulmonary outflow tract demonstrates peak Doppler velocity of approximately 5 m/sec suggesting significant obstruction. MLV, morphological left ventricle. Reproduced from Yarrabolu T.R., et al. [
23].
Figure 20.
(
A) Selected video frame from a subcostal four chamber view (similar to
Figure 19B) with color-Doppler demonstrating turbulent flow (TF) in the pulmonary outflow tract. (
B) Continuous wave Doppler recording across the pulmonary outflow tract demonstrates peak Doppler velocity of approximately 5 m/sec suggesting significant obstruction. MLV, morphological left ventricle. Reproduced from Yarrabolu T.R., et al. [
23].
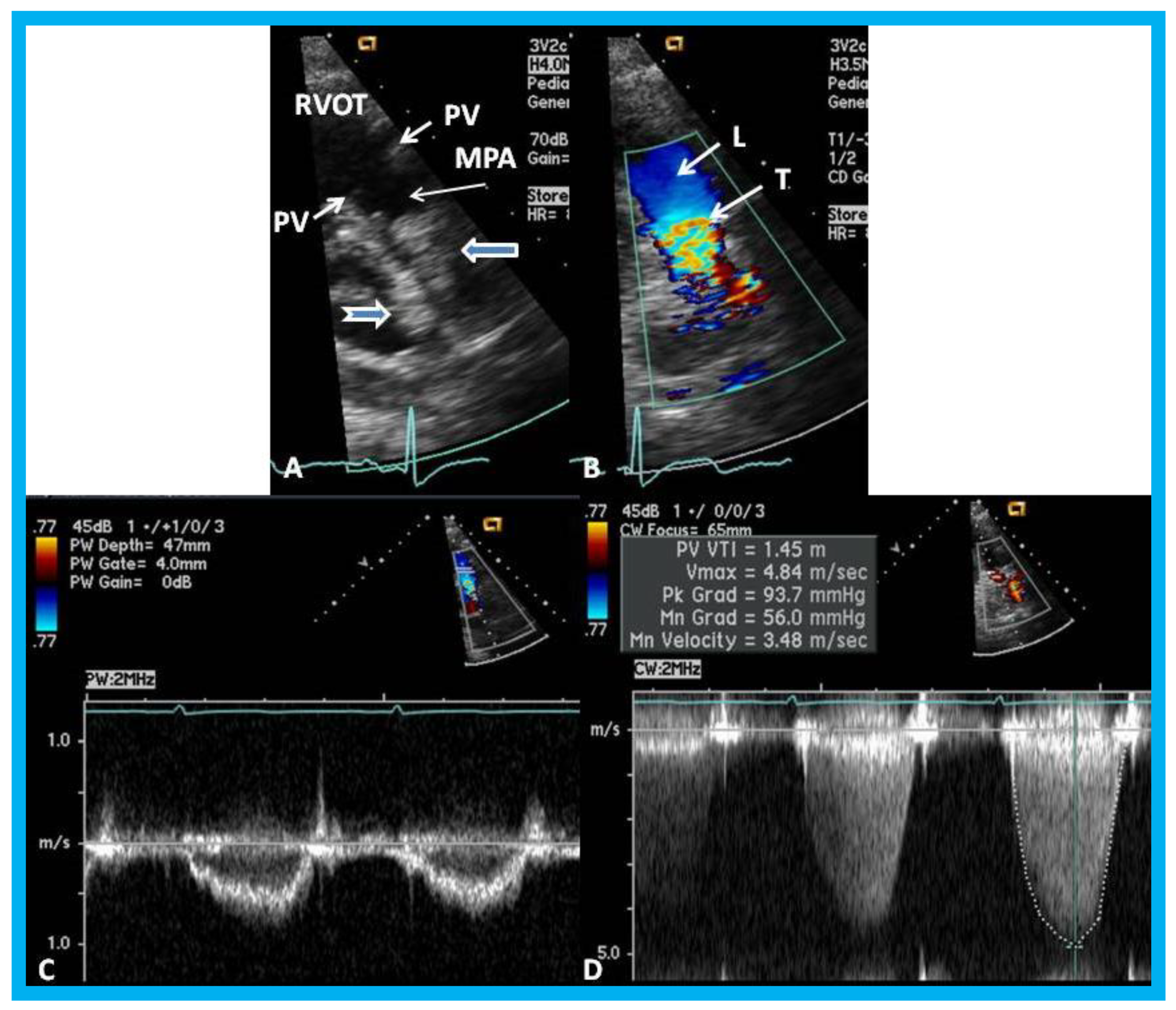
Figure 21.
(
A) Selected video frame from a parasternal short axis view showing echo dense structures (thick blue arrows) within and outside the main pulmonary artery (MPA): Pulmonary valve (PV) leaflets (small arrows) are shown and appear normal. The right ventricular outflow tract (RVOT) and proximal MPA are free of any echo-dense structures. (
B) Color-Doppler mapping of the same structures as in panel A shows normal laminar (L) flow in the RVOT and proximal MPA and turbulent (T) flow starting in the proximal MPA, indicating obstruction. (
C) Pulse Doppler sampling from the proximal MPA, which shows normal flow velocity. (
D) Continuous wave Doppler sampling demonstrating high velocity flow across the MPA with a calculated peak instantaneous gradient of 93.7 mmHg and a mean gradient of 56 mmHg, indicating severe obstruction. Reproduced from Mazur L., et al. [
31].
Figure 21.
(
A) Selected video frame from a parasternal short axis view showing echo dense structures (thick blue arrows) within and outside the main pulmonary artery (MPA): Pulmonary valve (PV) leaflets (small arrows) are shown and appear normal. The right ventricular outflow tract (RVOT) and proximal MPA are free of any echo-dense structures. (
B) Color-Doppler mapping of the same structures as in panel A shows normal laminar (L) flow in the RVOT and proximal MPA and turbulent (T) flow starting in the proximal MPA, indicating obstruction. (
C) Pulse Doppler sampling from the proximal MPA, which shows normal flow velocity. (
D) Continuous wave Doppler sampling demonstrating high velocity flow across the MPA with a calculated peak instantaneous gradient of 93.7 mmHg and a mean gradient of 56 mmHg, indicating severe obstruction. Reproduced from Mazur L., et al. [
31].
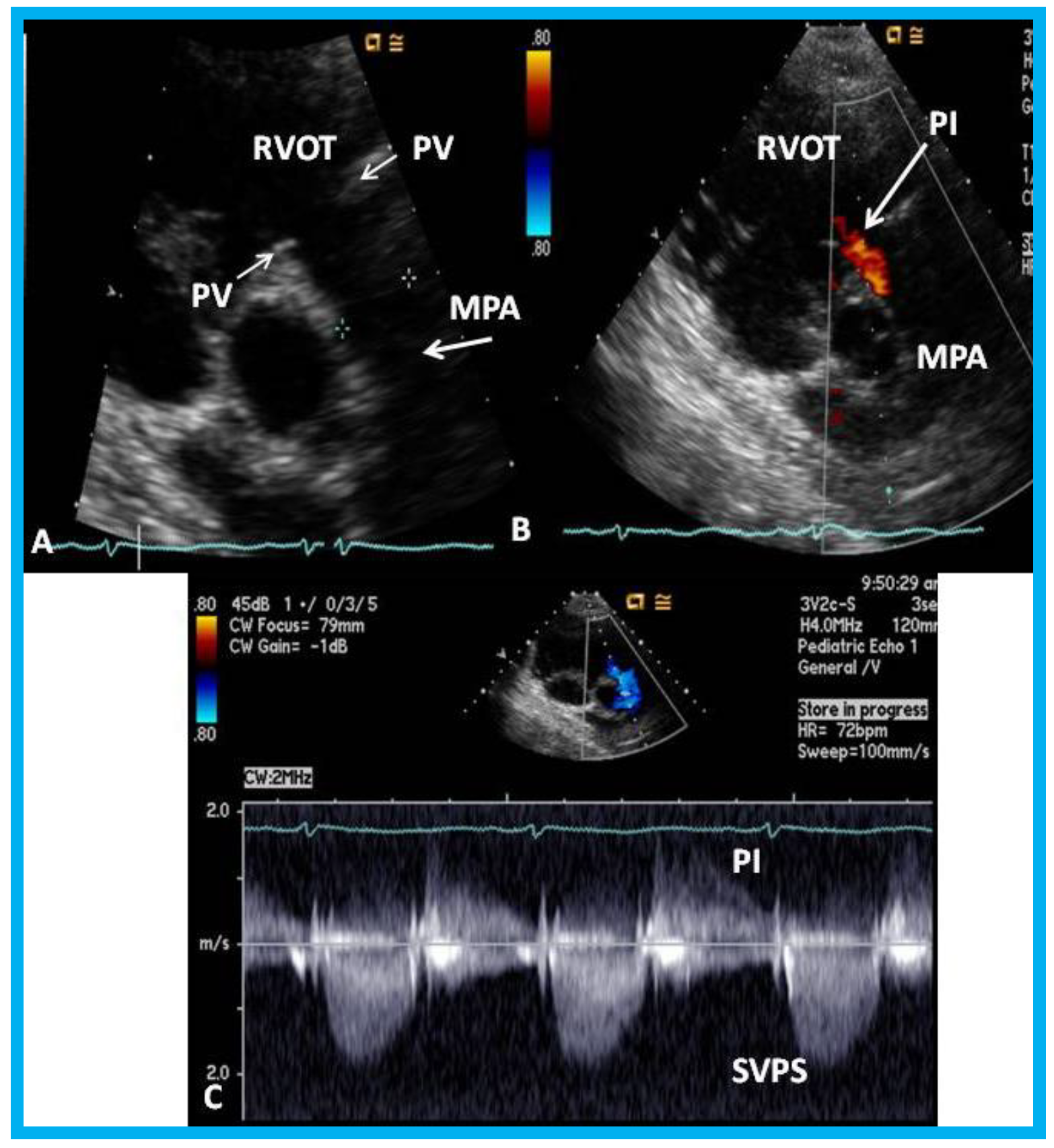
Figure 22.
Echo-Doppler studies performed five months after removal of the Nuss bar: (
A) Selected video frame from a parasternal short axis view demonstrating no echo dense structures in the right ventricular outflow tract (RVOT) and main pulmonary artery (MPA) that was seen prior to removal of the Nuss bar (
Figure 20A). Pulmonary valve (PV) leaflets (arrows) are shown. (
B) Color-Doppler mapping of the same structures as in panel A shows mild pulmonary insufficiency (PI) (arrow). (
C) Continuous wave Doppler sampling demonstrating low Doppler flow velocity across the MPA with a calculated peak instantaneous gradient of 15 mmHg, indicating minimal supravalvular pulmonary stenosis (SVPS) and mild pulmonary insufficiency (PI). Reproduced from Mazur L., et al. [
31].
Figure 22.
Echo-Doppler studies performed five months after removal of the Nuss bar: (
A) Selected video frame from a parasternal short axis view demonstrating no echo dense structures in the right ventricular outflow tract (RVOT) and main pulmonary artery (MPA) that was seen prior to removal of the Nuss bar (
Figure 20A). Pulmonary valve (PV) leaflets (arrows) are shown. (
B) Color-Doppler mapping of the same structures as in panel A shows mild pulmonary insufficiency (PI) (arrow). (
C) Continuous wave Doppler sampling demonstrating low Doppler flow velocity across the MPA with a calculated peak instantaneous gradient of 15 mmHg, indicating minimal supravalvular pulmonary stenosis (SVPS) and mild pulmonary insufficiency (PI). Reproduced from Mazur L., et al. [
31].
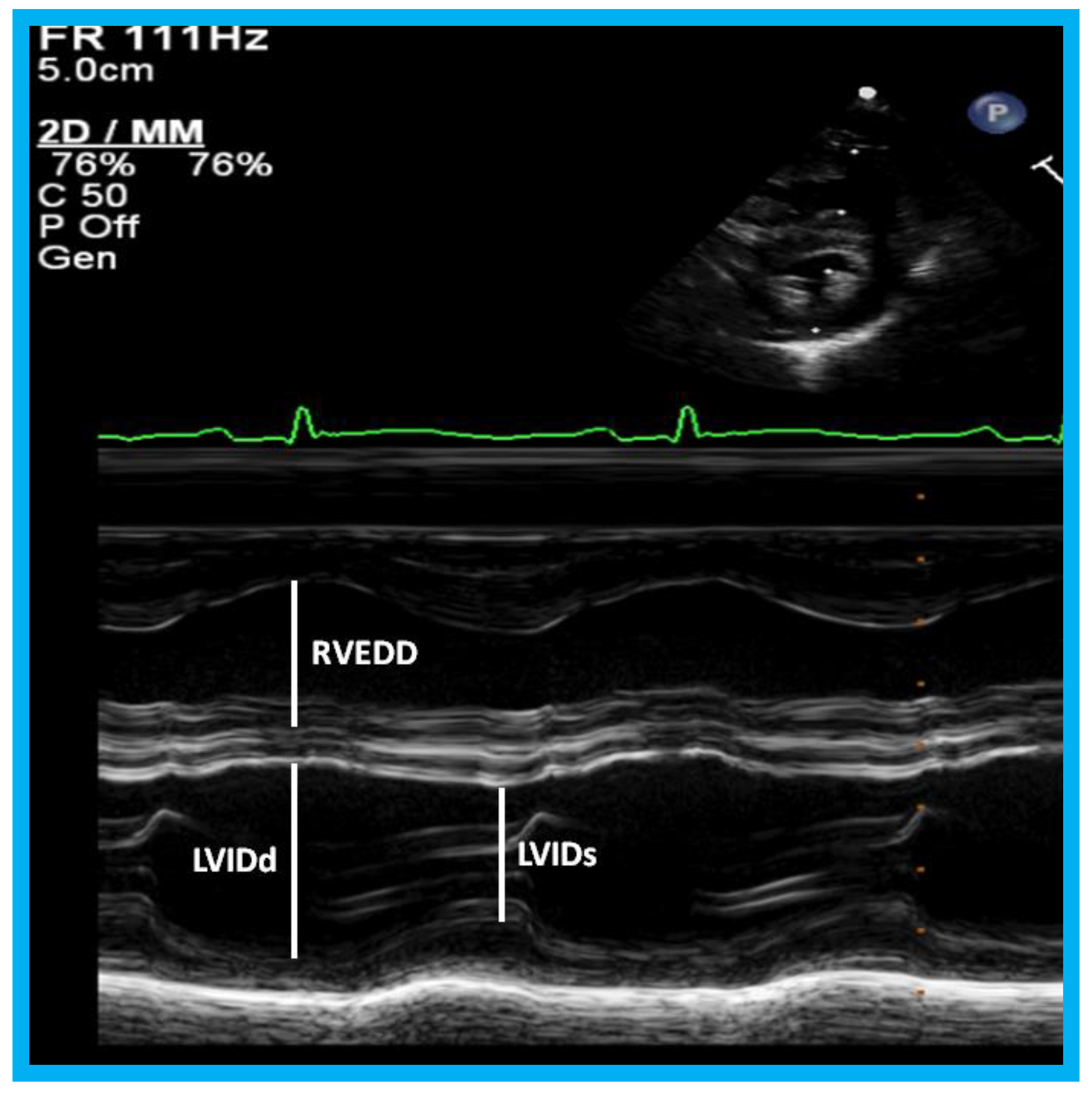
Figure 23.
M-mode recording of the ventricles demonstrating measurements of left ventricular internal dimension in end-diastole (LVIDd) and right ventricular end-diastolic dimension (RVEDD), both measured at the onset of the QRS complex of the simultaneously recorded electrocardiogram (ECG): The left ventricular internal dimension in systole (LVIDs) is measured at end-systole. The data are compared with normal values, and z score is determined. These data are also used for calculation left ventricular fractional shortening: FS = {(LVIDd − LVIDs)/LVIDd} 100 (where FS is fractional shortening, LVIDd is left ventricular end-diastolic dimension, and LVIDs is left ventricular end-systolic dimension.) to assess the left ventricular systolic function.
Figure 23.
M-mode recording of the ventricles demonstrating measurements of left ventricular internal dimension in end-diastole (LVIDd) and right ventricular end-diastolic dimension (RVEDD), both measured at the onset of the QRS complex of the simultaneously recorded electrocardiogram (ECG): The left ventricular internal dimension in systole (LVIDs) is measured at end-systole. The data are compared with normal values, and z score is determined. These data are also used for calculation left ventricular fractional shortening: FS = {(LVIDd − LVIDs)/LVIDd} 100 (where FS is fractional shortening, LVIDd is left ventricular end-diastolic dimension, and LVIDs is left ventricular end-systolic dimension.) to assess the left ventricular systolic function.
Figure 24.
Apical four-chamber views of the left ventricle (LV) in end-diastole (LVED) in (A) and end-systole (LVES) in (B) demonstrating calculation of area shortening of the LV using Simpson’s rule: AS = (LVAd − LVAs)/LVAd (where AS is area shortening, LVAd is LV area in diastole, and LVAs is LV area in systole). The LV area shortening is 52% (see insert in (B)); normal values are 40 to 60%.
Figure 24.
Apical four-chamber views of the left ventricle (LV) in end-diastole (LVED) in (A) and end-systole (LVES) in (B) demonstrating calculation of area shortening of the LV using Simpson’s rule: AS = (LVAd − LVAs)/LVAd (where AS is area shortening, LVAd is LV area in diastole, and LVAs is LV area in systole). The LV area shortening is 52% (see insert in (B)); normal values are 40 to 60%.
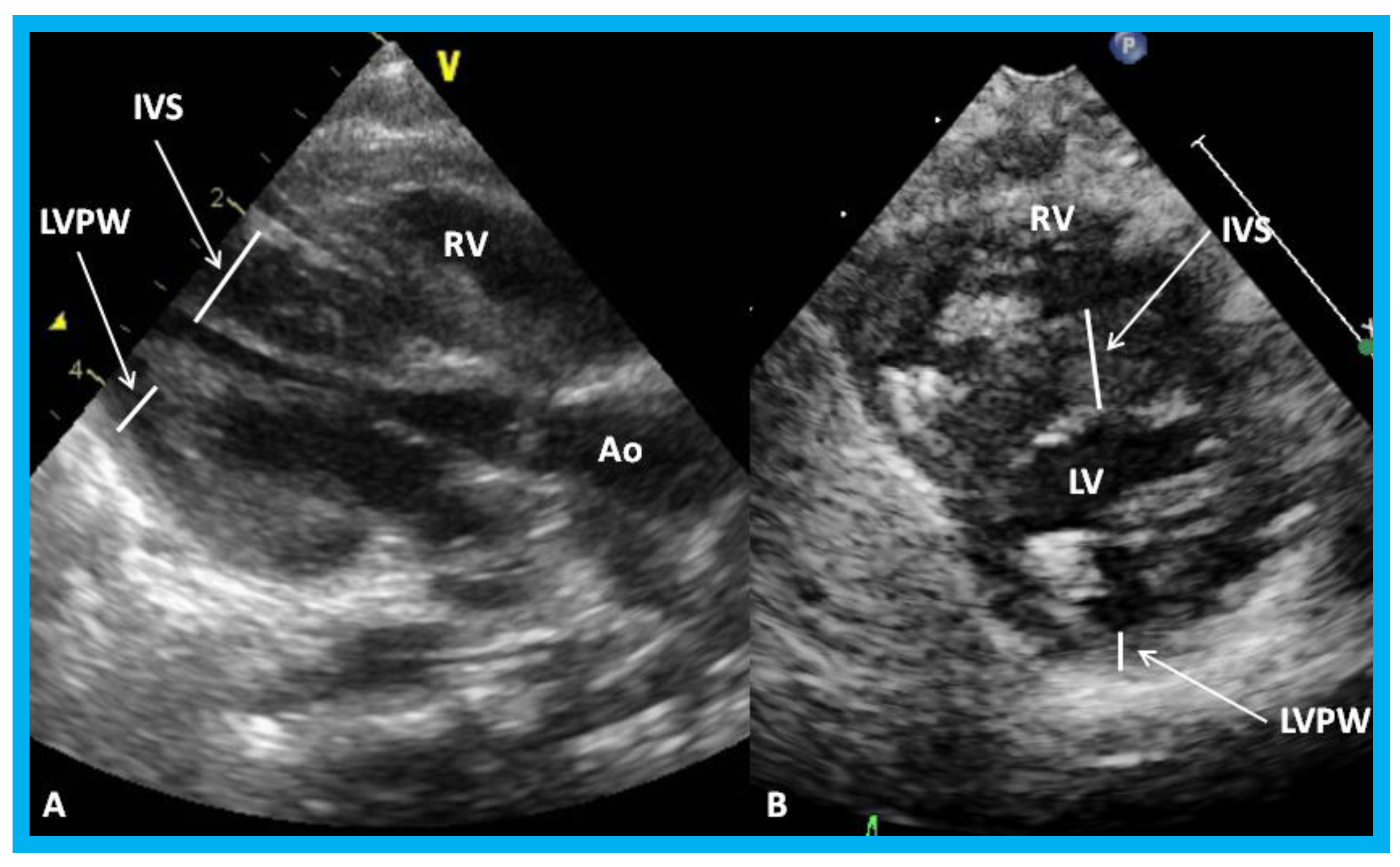
Figure 25.
Parasternal long (A) and short (B) axis views of the left ventricle (LV) demonstrating markedly thickened interventricular septum (IVS) in an infant of a diabetic mother. Ao, aorta; LVPW, LV posterior wall thickness; RV, right ventricle.
Figure 25.
Parasternal long (A) and short (B) axis views of the left ventricle (LV) demonstrating markedly thickened interventricular septum (IVS) in an infant of a diabetic mother. Ao, aorta; LVPW, LV posterior wall thickness; RV, right ventricle.
Figure 26.
(A,B). Subcostal views of the left ventricle (LV) in an infant of a diabetic mother showing complete obliteration of the LV cavity in systole (arrow in (B)).
Figure 26.
(A,B). Subcostal views of the left ventricle (LV) in an infant of a diabetic mother showing complete obliteration of the LV cavity in systole (arrow in (B)).
Figure 27.
(A) Parasternal long axis, two-dimensional, and color-Doppler images demonstrating turbulent flow (TF) in the outflow tract of the left ventricle (LV) in an infant of a diabetic mother. (B) Continuous wave Doppler recording demonstrates a peak instantaneous gradient of 39 mmHg (see the insert in (B)): Note the triangular pattern of the Doppler recording indicative of subaortic obstruction. Ao, Aorta.
Figure 27.
(A) Parasternal long axis, two-dimensional, and color-Doppler images demonstrating turbulent flow (TF) in the outflow tract of the left ventricle (LV) in an infant of a diabetic mother. (B) Continuous wave Doppler recording demonstrates a peak instantaneous gradient of 39 mmHg (see the insert in (B)): Note the triangular pattern of the Doppler recording indicative of subaortic obstruction. Ao, Aorta.
Figure 28.
High parasternal views of the right innominate (RInn) artery demonstrating its division into right common carotid (RCC) artery and right subclavian (RS) artery by 2D (A) and color flow imaging (B): These data suggest left aortic arch.
Figure 28.
High parasternal views of the right innominate (RInn) artery demonstrating its division into right common carotid (RCC) artery and right subclavian (RS) artery by 2D (A) and color flow imaging (B): These data suggest left aortic arch.
Figure 29.
Apical four-chamber view of the heart in baby with Down syndrome demonstrating atrioventricular septal defect in 2D (A) and color-Doppler imaging (B): Ostium primum atrial septal defect (PASD) and ventricular septal defect (VSD) are shown (arrows) in (A) and mitral regurgitation (MR) in (B) (arrow). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Figure 29.
Apical four-chamber view of the heart in baby with Down syndrome demonstrating atrioventricular septal defect in 2D (A) and color-Doppler imaging (B): Ostium primum atrial septal defect (PASD) and ventricular septal defect (VSD) are shown (arrows) in (A) and mitral regurgitation (MR) in (B) (arrow). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Figure 30.
Apical four-chamber (A,B) and parasternal short axis (C) views of the heart in an infant with cardiomegaly showing a significant pericardial effusion (thick arrows): Fibrin strands in the effusion are shown (thin arrows).
Figure 30.
Apical four-chamber (A,B) and parasternal short axis (C) views of the heart in an infant with cardiomegaly showing a significant pericardial effusion (thick arrows): Fibrin strands in the effusion are shown (thin arrows).
Figure 31.
Parasternal long (A) and short (B) axis views of the heart in a baby with cardiomegaly demonstrating a large pericardial effusion (arrows).
Figure 31.
Parasternal long (A) and short (B) axis views of the heart in a baby with cardiomegaly demonstrating a large pericardial effusion (arrows).
Figure 32.
Parasternal short axis echo-Doppler imaging (A) demonstrating patent ductus arteriosus (PDA) with left-to-right shunt and high Doppler flow velocity (B) across the PDA suggestive of normal/low pulmonary artery pressure. Ao, aorta; D, diastolic; DAo, descending aorta; RV, right ventricle; S, systolic.
Figure 32.
Parasternal short axis echo-Doppler imaging (A) demonstrating patent ductus arteriosus (PDA) with left-to-right shunt and high Doppler flow velocity (B) across the PDA suggestive of normal/low pulmonary artery pressure. Ao, aorta; D, diastolic; DAo, descending aorta; RV, right ventricle; S, systolic.
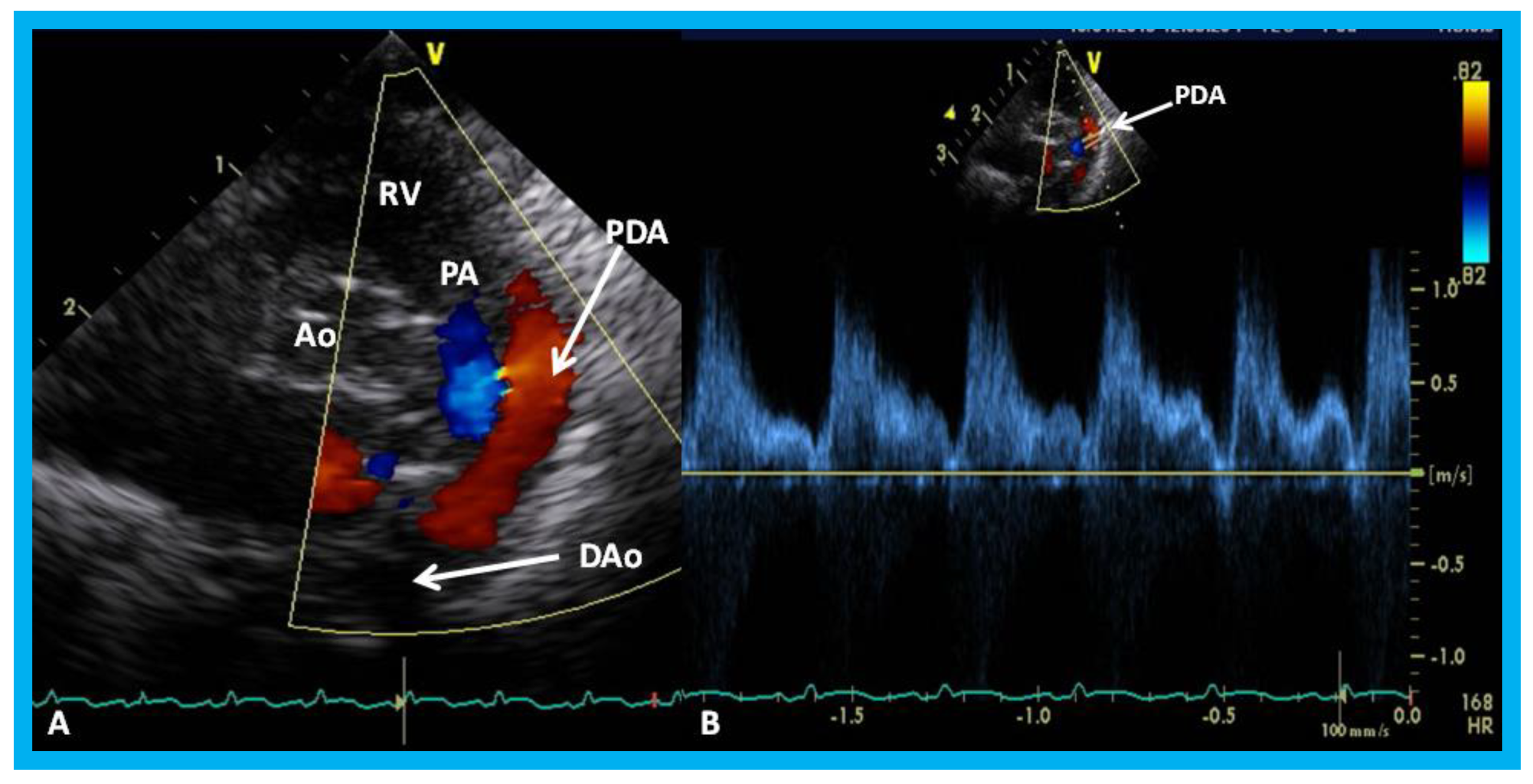
Figure 33.
Parasternal short axis echo-Doppler imaging (A) demonstrating patent ductus arteriosus (PDA) with left-to-right shunt and low Doppler velocity (B) across the PDA suggestive of elevated pulmonary artery pressure. Ao, aorta; DAo, descending aorta; PA, pulmonary artery; RV, right ventricle.
Figure 33.
Parasternal short axis echo-Doppler imaging (A) demonstrating patent ductus arteriosus (PDA) with left-to-right shunt and low Doppler velocity (B) across the PDA suggestive of elevated pulmonary artery pressure. Ao, aorta; DAo, descending aorta; PA, pulmonary artery; RV, right ventricle.
Figure 34.
(A) Echocardiographic frame from a suprasternal notch view illustrating laminar flow in the descending aorta (DAo) in a premature infant with a small ductus (not shown). (B) Continuous wave Doppler recording in the same infant shows normal systolic flow (SF) (*) and normal anterograde diastolic flow (ADF) in the DAo; the diastolic flow is seen below the baseline.
Figure 34.
(A) Echocardiographic frame from a suprasternal notch view illustrating laminar flow in the descending aorta (DAo) in a premature infant with a small ductus (not shown). (B) Continuous wave Doppler recording in the same infant shows normal systolic flow (SF) (*) and normal anterograde diastolic flow (ADF) in the DAo; the diastolic flow is seen below the baseline.
Figure 35.
Doppler interrogation from suprasternal notch position in two different premature infants (A,B) with large patent ductus arteriosus (PDA) show retrograde diastolic flow (RDF) in the descending aorta (DAo), suggesting that these PDAs are likely be hemodynamically significant PDAs. Low magnitude systolic flow (SF) (*) suggests that there is no evidence for descending aortic obstruction.
Figure 35.
Doppler interrogation from suprasternal notch position in two different premature infants (A,B) with large patent ductus arteriosus (PDA) show retrograde diastolic flow (RDF) in the descending aorta (DAo), suggesting that these PDAs are likely be hemodynamically significant PDAs. Low magnitude systolic flow (SF) (*) suggests that there is no evidence for descending aortic obstruction.
Table 1.
Comparative minimal ductal diameters data of various age groups.
Table 1.
Comparative minimal ductal diameters data of various age groups.
| Age in Months | TD MDD (mm) | Color-Doppler MDD (mm) | Angiographic MDD (mm) * |
|---|
| All patients: 4 to 303 months | 3.99 ± 1.37 | 4.26 ± 1.57 | 2.28 ± 1.25 |
| 4 to 15 months | 4.27 ± 1.36 | 5.23 ± 1.80 | 2.91 ± 1.50 |
| 16 to 30 months | 3.49 ± 1.06 | 4.17 ± 1.61 | 1.89 ± 0.97 |
| 31 to 45 months | 4.03 ± 2.04 | 4.07 ± 1.72 | 1.90 ± 1.19 |
| 46 to 60 months | 3.76 ± 1.85 | 3.23 ± 1.22 | 1.46 ± 0.65 |
| 61 to 75 months | 3.98 ± 1.36 | 3.83 ± 1.29 | 3.26 ± 1.69 |
| 76 to 303 months | 4.12 ± 1.25 | 4.14 ± 1.34 | 2.05 ± 0.83 |
Table 2.
Characterization size of Patent Ductus Arteriosus (PDA) in premature infants by echo-Doppler studies.
Table 2.
Characterization size of Patent Ductus Arteriosus (PDA) in premature infants by echo-Doppler studies.
| Parameter | Small PDA | Moderate PDA * | Large PDA * |
|---|
| Size of the LA | Normal | Mildly dilated | Moderate to severely dilated |
| LA:Ao Ratio | ≤1.4:1 | 1.4 to 1.6 | ≥1.6 |
| Size of the LV | Normal | Mildly dilated | Moderate to severely dilated |
| Systolic Function of the LV | Normal | Normal | Normal, hyper-contractile or diminished function |
| Estimated PA Pressure | Normal | Mildly elevated | Moderate to severely elevated |
| Minimal Diameter of the PDA | ≤1.4 mm | 1.4 to 2.0 mm | ≥2.0 mm |
| Doppler Velocity across the PDA | High (3.0 to 4.0 m/s) | ~2.0 m/s | Low (~1.0 m/s) |
| Descending Aortic Doppler Flow Velocity Pattern | Normal anterograde flow (Figure 33) | Normal anterograde flow (Figure 33) | Normal or absent anterograde flow or presence of retrograde flow (Figure 34) |