Robotic Stereotactic Assistance (ROSA) for Pediatric Epilepsy: A Single-Center Experience of 23 Consecutive Cases
Abstract
1. Introduction
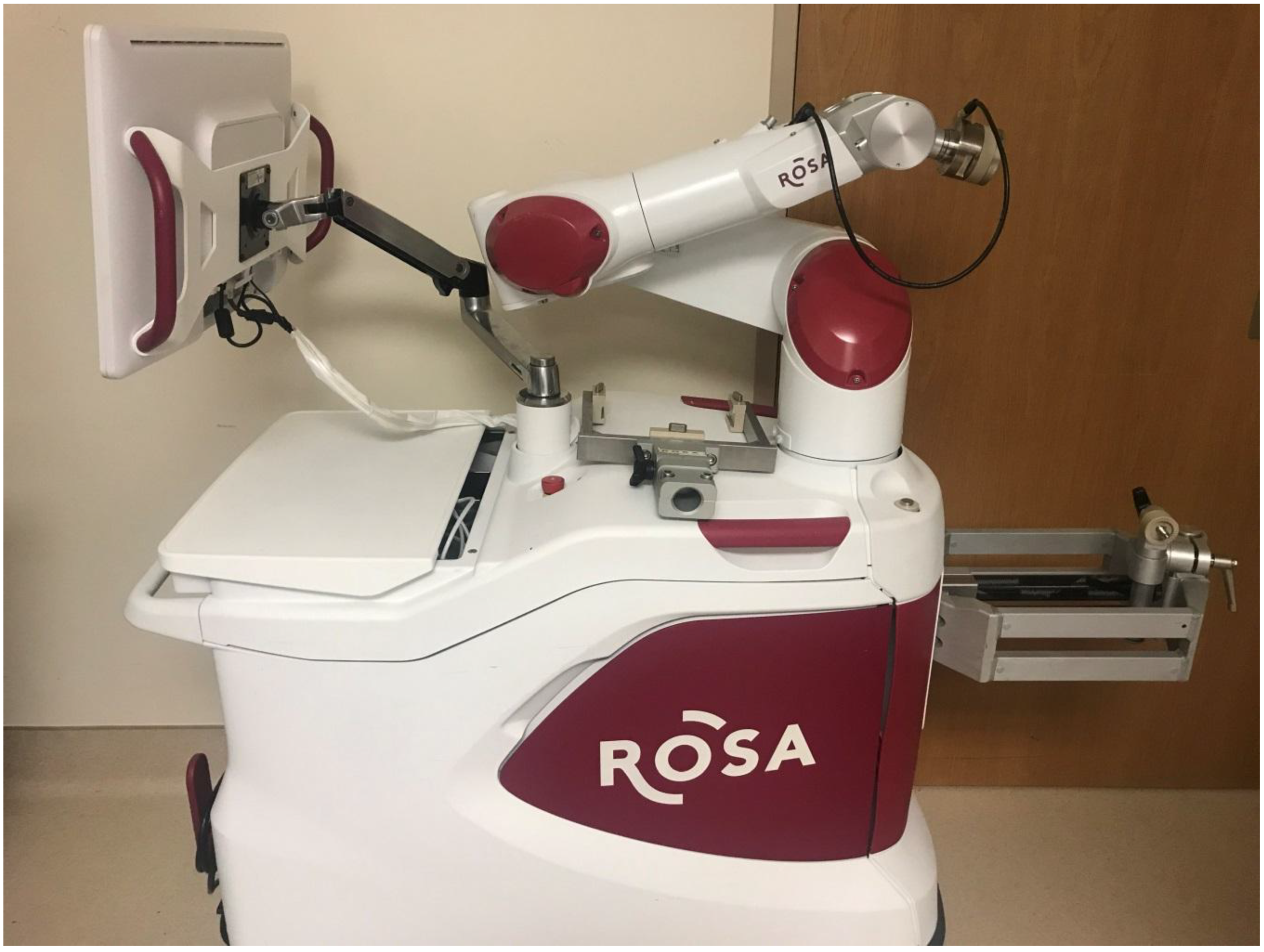
2. Materials and Methods
3. Results
3.1. Anesthetic Management
3.1.1. Preoperative Evaluation, Induction, and Maintenance
3.1.2. Positioning and Protection
3.1.3. Hemodynamic Control and Ventilation Strategies
3.1.4. Emergence and Postoperative Care
3.2. Surgical Management
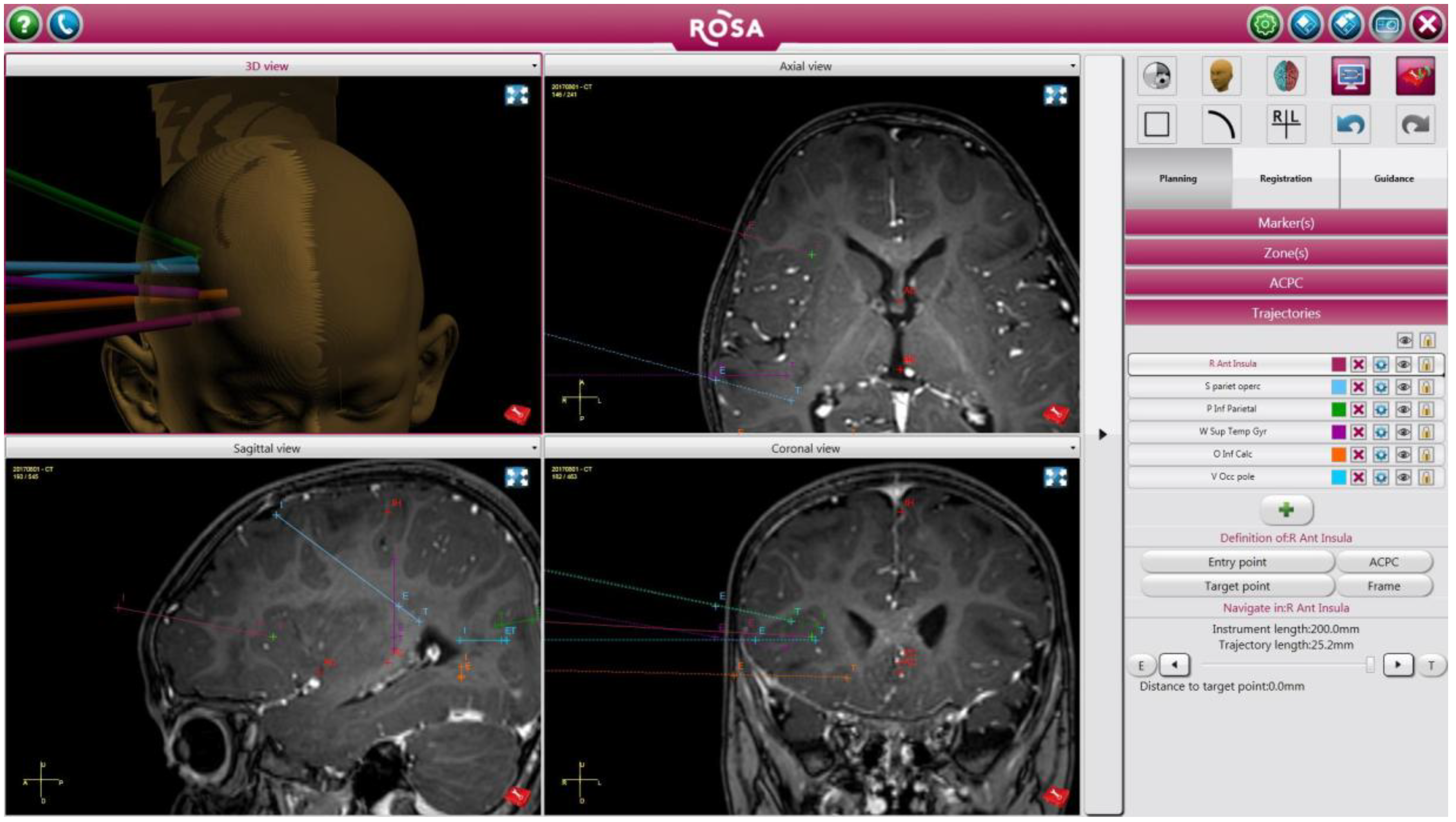
3.3. Illustrative Case: Patient 10
3.3.1. Preoperative Evaluation
3.3.2. Anesthetic Management
3.3.3. Operative Details
3.3.4. Postoperative Course
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miller, B.; Salehi, A.; Limbrick, D.; Smyth, M. Applications of a robotic stereotactic arm for pediatric epilepsy and neurooncology surgery. J. Neurosurg. Pediatr. 2017, 20, 364–370. [Google Scholar] [CrossRef] [PubMed]
- De Benedictis, A.; Trezza, A.; Carai, A.; Genovese, E.; Procaccini, E.; Messina, R.; Randi, F.; Cossu, S.; Esposito, G.; Palma, P.; et al. Robot-assisted procedures in pediatric neurosurgery. Neurosurg. Focus 2017, 42, E7. [Google Scholar] [CrossRef] [PubMed]
- Hoshide, R.; Calayag, M.; Meltzer, H.; Levy, M.L.; Gonda, D. Robot-assisted endoscopic third ventriculostomy: Institutional experience in 9 patients. J. Neurosurg. Pediatr. 2017, 20, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martinez, J.; Vadera, S.; Mullin, J.; Enatsu, R.; Alexopoulos, A.V.; Patwardhan, R.; Bingaman, W.; Najm, I. Robot-assisted stereotactic laser ablation in medically intractable epilepsy: Operative technique. Neurosurgery 2014, 10 (Suppl. 2), 167–172. [Google Scholar] [CrossRef] [PubMed]
- Taussig, D.; Chipaux, M.; Lebas, A.; Fohlen, M.; Bulteau, C.; Turnier, J.; Ferrand-Sorbets, S.; Dalalande, O.; Dorfmuller, G. Stereo-electroencephalography (SEEG) in 65 children: An effective and safe diagnostic method for pre-surgical diagnosis, independent of age. Epileptic Disord. 2014, 16, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Carai, A.; Mastronuzzi, A.; De Benedictis, A.; Messina, R.; Cacchione, A.; Miele, E.; Randi, F.; Esposito, G.; Trezza, A.; Colafati, G.S.; et al. Robot-Assisted Stereotactic Biopsy of Diffuse Intrinsic Pontine Glioma: A Single Center Experience. World Neurosurg. 2017, 101, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martinez, J.; Bulacio, J.; Thompson, S.; Gale, J.; Smithason, S.; Najm, I.; Bingaman, W. Technique, Results, and Complications Related to Robot-Assisted Stereoelectroencephalography. Neurosurgery 2016, 78, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Chui, J.; Manninen, P.; Valiante, T.; Venkatraghavan, L. The Anesthetic Considerations of Intraoperative Electrocorticography during Epilepsy Surgery. Anesth. Analg. 2013, 117, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Soriano, S.G.; Eldredge, E.A.; Wang, F.K.; Kull, L.; Madsen, J.R.; Black, P.M.; Riviello, J.J.; Rockoff, M.A. The effect of propofol on intraoperative electrocorticography and cortical stimulation during awake craniotomy in children. Paediatr. Anaesth. 2000, 10, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Hisada, K.; Morioka, T.; Fukui, K.; Nishio, S.; Kuruma, T.; Irita, K.; Takahashi, S.; Fukui, M. Effects of Sevoflurane and Isoflurane on Electrocorticographic Activities in Patients with Temporal Lobe Epilepsy. J. Neurosurg. Anesthesiol. 2001, 13, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Hosain, S.; Nagarajan, L.; Fraser, R.; Van Poznak, A.; Labar, D. Effects of nitrous oxide on electrocorticography during epilepsy surgery. Electroencephalogr. Clin. Neurophysiol. 1997, 102, 340–342. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, J.; Mullin, J.; Vadera, S.; Bulacio, J.; Hughes, G.; Jones, S.; Enatsu, R.; Najm, I. Stereotactic placement of depth electrodes in medically intractable epilepsy. J. Neurosurg. 2014, 120, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martinez, J.; Mullin, J.; Bulacio, J.; Gupta, A.; Enatsu, R.; Najm, I.; Bingaman, W.; Wyllie, E.; Lachhwani, D. Stereoelectroencephalography in children and adolescents with difficult-to-localize refractory focal epilepsy. Neurosurgery 2014, 75, 258–268. [Google Scholar] [CrossRef] [PubMed]
| Characteristic/Outcome | N (%) | Mean (SD) | Median (Range) |
|---|---|---|---|
| Age (years) | - | 13 (5) | 14 (2–21) |
| Weight (kg) | - | 52 (21) | 54 (14–102) |
| Sex | |||
| male | 17 (74) | - | - |
| female | 6 (26) | - | - |
| Induction of anesthesia | |||
| Inhalational (8% sevoflurane + 70% nitrous oxide) | 18 (78) | - | - |
| Intravenous (2% lidocaine 1 mg/kg + propofol 2–3 mg/kg) | 5 (22) | - | - |
| Vascular access | |||
| 2 peripheral IV catheters | 23 (100) | - | - |
| radial arterial line | 7 (30) | - | - |
| Maintenance of anesthesia | |||
| sevoflurane (MAC 0.5) | 20 (87) | - | - |
| isoflurane (MAC 0.5) | 3 (13) | - | - |
| remifentanil (0.1–1 µg/kg/min) | 13 (57) | - | - |
| sufentanil (0.1–0.25 µg/kg/h) | 1 (4) | - | - |
| no continuous opioid infusion | 9 (39) | - | - |
| fentanyl (µg/kg) | 22 (96) | 2.2 (1.4) | 1.9 (0–4.4) |
| morphine (mg/kg) | 7 (30) | 0.02 (0.04) | 0 (0–0.14) |
| rocuronium used | 18 (78) | - | - |
| Vasopressors | |||
| none | 18 (78) | - | - |
| ephedrine (0.04–0.15 mg/kg) | 5 (22) | - | - |
| Fluid administration | |||
| crystalloid (mL/kg) | 23 (100) | 25 (10) | 27 (8–46) |
| 5% albumin (mL/kg) | 7 (30) | 2 (4) | 0 (0–11) |
| blood product transfusion (mL/kg) | 1 (4) | 0.2 (0.9) | 0 (0–4.4) |
| Urine output (mL/kg) | 23 (100) | 4 (3) | 3 (0.4–11.5) |
| Extubated at end of surgery | |||
| no | 0 (0) | - | - |
| yes | 23 (100) | - | - |
| Operative time (minutes) | - | 148 (60) | 129 (67–252) |
| Number of SEEG electrodes placed | 148 | 6 (3) | 6 (2–13) |
| Complications | |||
| intraoperative | 0 (0) | - | - |
| postoperative | 0 (0) | - | - |
| Postoperative CT | 18 (78) | - | - |
| PICU length of stay (days) | - | 1 (0) | 1 (1–1) |
| Total hospital length of stay (days) | - | 9 (6) | 8 (2–29) |
| Patient Number | Age (Years) | Weight (kg) | Sex | Diagnosis | Prior Neurosurgical Intervention | Number of Electrodes Placed | Operative Time (Minutes) | Hospital [PICU] Length of Stay (Days) | Postoperative Imaging | Subsequent Surgery Based on SEEG Results | Seizure Outcome after Resective/Ablative Surgery |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 17 | 102 | M | epilepsy | none | 4 | 116 | 8 [1] | CT | none | N/A |
| 2 | 14 | 53 | M | epilepsy, DEPDC5/HNRNHP1 genes | none | 10 | 245 | 22 [1] | CT | none | N/A |
| 3 | 8 | 35 | M | epilepsy | anaplastic ependymoma resection | 9 | 104 | 11 [1] | CT | seizure focus resection | seizure free |
| 4 | 14 | 71 | M | epilepsy | none | 8 | 158 | 10 [1] | CT | NeuroPace, DBS pending | N/A |
| 5 | 21 | 54 | M | epilepsy | cortical dysplasia excision ×2 | 8 | 201 | 8 [1] | CT | Visualase with ClearPoint | seizure free |
| 6 | 15 | 75 | M | epilepsy | none | 13 | 228 | 11 [1] | CT | none, NeuroPace pending | N/A |
| 7 | 14 | 75 | F | epilepsy | VNS, brain biopsy | 9 | 136 | 13 [1] | CT | NeuroPace, DBS | ↓ seizure frequency |
| 8 | 6 | 23 | M | tuberous sclerosis, epilepsy | tuber resection | 8 | 99 | 8 [1] | CT | Visualase | unchanged |
| 6 | 3 * | 252 | 2 [1] | iMRI | none | ||||||
| 23 | |||||||||||
| 9 | 17 | 55 | M | epilepsy | frontal lobe seizure focus resection | 5 | 80 | 7 [1] | CT | Visulase | unchanged |
| 17 | 2 * | 152 | 2 [1] | iMRI | none | ||||||
| 55 | |||||||||||
| 10 | 12 | 58 | F | epilepsy | frontal cortical dysplasia excision ×3 | 4 | 67 | 11 [1] | CT | Visualase | ↓ seizure frequency |
| 12 | 2 * | 209 | 2 [1] | iMRI | none | ||||||
| 58 | |||||||||||
| 11 | 16 | 74 | M | epilepsy | none | 12 | 129 | 12 [1] | CT | grid placement, partial resection of temporal lobe, right amygdalohippocampectomy | ↓ seizure frequency |
| 12 | 12 | 52 | F | Aicardi syndrome, epilepsy | none | 2 * | 186 | 2 [1] | iMRI | none | N/A |
| 13 | 2 | 14 | M | tuberous sclerosis, epilepsy | none | 5 | 124 | 9 [1] | CT | resection of right inferiomedial frontal calcified tumor | unchanged |
| 14 | 15 | 54 | M | epilepsy | right frontal lobectomy | 6 | 91 | 8 [1] | CT | Visualase | ↓ seizure frequency |
| 2 * | 216 | 2 [1] | iMRI | none | |||||||
| 15 | 20 | 56 | M | epilepsy | none | 5 | 71 | 12 [1] | CT | temporal lobectomy | seizure free |
| 16 | 14 | 46 | F | epilepsy | temporal lobectomy | 4 | 100 | 14 [1] | CT | left temporal lobectomy, left hippocampectomy | seizure free |
| 17 | 8 | 81 | M | epilepsy | none | 10 | 225 | 6 [1] | CT | grid placement and removal, NeuroPace | N/A |
| 18 | 7 | 23 | M | oligodendroglioma | tumor resection ×3 | 7 | 89 | 29 [1] | CT | none | N/A |
| 19 | 12 | 55 | F | epilepsy | seizure focus resection | 10 | 124 | 8 [1] | CT | none | N/A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelson, J.H.; Brackett, S.L.; Oluigbo, C.O.; Reddy, S.K. Robotic Stereotactic Assistance (ROSA) for Pediatric Epilepsy: A Single-Center Experience of 23 Consecutive Cases. Children 2020, 7, 94. https://doi.org/10.3390/children7080094
Nelson JH, Brackett SL, Oluigbo CO, Reddy SK. Robotic Stereotactic Assistance (ROSA) for Pediatric Epilepsy: A Single-Center Experience of 23 Consecutive Cases. Children. 2020; 7(8):94. https://doi.org/10.3390/children7080094
Chicago/Turabian StyleNelson, Jonathon H., Samantha L. Brackett, Chima O. Oluigbo, and Srijaya K. Reddy. 2020. "Robotic Stereotactic Assistance (ROSA) for Pediatric Epilepsy: A Single-Center Experience of 23 Consecutive Cases" Children 7, no. 8: 94. https://doi.org/10.3390/children7080094
APA StyleNelson, J. H., Brackett, S. L., Oluigbo, C. O., & Reddy, S. K. (2020). Robotic Stereotactic Assistance (ROSA) for Pediatric Epilepsy: A Single-Center Experience of 23 Consecutive Cases. Children, 7(8), 94. https://doi.org/10.3390/children7080094





