Secular Changes in Body Build and Body Composition in Czech Preschool Children in the Context of Latent Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurements
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Commission on Ending Childhood Obesity: Fact and Figures on Childhood Obesity; WHO: Geneva, Swizerland, 2019; Available online: https://www.who.int/end-childhood-obesity/en/ (accessed on 23 July 2020).
- Olds, T.; Tomkinson, G.; Léger, C.; Cazorla, G. Worldwide variation in the performance of children and adolescence: An analysis of 109 studies of the 20-m shuttle run test in 37 countries. J. Sports Sci. 2006, 24, 1025–1038. [Google Scholar] [CrossRef]
- Kohl, H.W.; Craig, C.L.; Lambert, E.V.; Inoue, S.; Alkandari, J.R.; Leetongin, G.; Kahlmeier, S.; Lancet Physical Activity Series Working Group. The pandemic of physical inactivity:global action for public health. Lancet 2012, 380, 294–305. [Google Scholar] [CrossRef]
- Pařízková, J. Impact of education on food behaviour, body composition, and physical fitness in children. Brit. J. Nutr. 2008, 99, S26–S32. [Google Scholar] [CrossRef]
- Pařízková, J. Nutrition, Physical Activity and Health in Early Life, 2nd ed.; CRC Press, Taylor and Francis Group: London, UK; New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Elizondo-Montemayor, L.; Serrano-Gonzalez, M.; Ugalde-Casas, P.A.; Cuello-Garcia, C.; Borbolla-Escoboza, J.R. Metabolic syndrome risk factors among a sample of overweight and obese Mexican children. J. Clin. Hypertens. 2010, 12, 380–387. [Google Scholar] [CrossRef]
- Maligie, M.; Crume, T.; Scherzinger, A.; Stamm, E.; Dabelea, D. Adiposity, fat patterning, and the metabolic syndrome among diverse youth: The EPOCH study. J. Pediatr. 2012, 161, 875–880. [Google Scholar] [CrossRef]
- Janz, K.F.; Kwon, S.; Letuchy, E.M.; Gilmore, J.M.E.; Burns, T.L.; Torner, J.C.; Willing, M.C.; Levy, S.M. Sustained effect of early physical activity on body fas mass on older children. Am. J. Prev. Med. 2009, 37, 35–40. [Google Scholar] [CrossRef]
- Sedlak, P.; Pařízková, J.; Daniš, R.; Dvořáková, H.; Vignerová, J. Secular changes of adiposity and motor development in Czech preschool children: Lifestyle changes in fifty-five year retrospective study. BioMed. Res. Int. 2015. [Google Scholar] [CrossRef]
- Mond, J.M.; Stich, H.; Hay, P.J.; Kraemer, A.; Baune, B.T. Association between obesity and developmental functioning in preschool children: A population-based study. Int. J. Obes. 2007, 31, 1068–1073. [Google Scholar] [CrossRef]
- Castlebon, K.; Andreeva, T. Obesity and motor skills among 4 to 6-year-old children in the United States: Nationally-representative surveys. BMC Pediatr. 2012, 12. [Google Scholar] [CrossRef]
- Kowal, M.; Kryst, L.; Woronkowicz, A.; Sobiecky, J. Long-term changes in body composition and prevalence of overweight and obesity in girls (aged 3–18 years) from Kraków (Poland) from 1883, 2000 and 2010. Ann. Hum. Biol. 2014, 41, 415–427. [Google Scholar] [CrossRef]
- Leppanen, M.H.; Nystrom, C.D.; Henriksson, P.; Pomeroy, J.; Ruiz, J.R.; Ortega, F.B.; Cadenas-Sánchez, C.; Löf, M. Physical activity intensity, sedentary behavior, body composition and fitness in 4-year-old children: Results from the ministop study. Int. J. Obes. 2016, 40, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Tellez, B.; Sanchez-Delgado, G.; Cadenaz-Sanchez, C.; Mora-Gonzalez, J.; Martín-Matillas, M.; Lof, M.; Ortega, F.B.; Ruiz, J.R. Health-related physical fitness is associated with total and central body fat in preschool children aged 3 to 5 years. Pediatr. Obes. 2016, 11, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Vale, S.; Trost, S.G.; Rêgo, C.; Abreu, S.; Mota, J. Physical activity, obesity status, and blood pressure in preschool children. J. Pediatr. 2015, 167, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Nyström, C.D.; Sandin, S.; Henriksson, P.; Trolle-Lagerros, Y.; Larsson, C.; Maddison, R.; Ortega, F.B.; Pomeroy, J.; Ruiz, J.R.; Silfvernagel, K.; et al. Mobile-based intervention intended to stop obesity in preschool-aged children: The MINISTOP randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 1327–1335. [Google Scholar] [CrossRef]
- Antunes, A.M.; Freitas, D.L.; Maia, J.; Hedeker, D.; Gouveia, E.R.; Thomis, M.; Lefevre, J.; Barnett, L.M. Motor performance, body fatness, and environmental factors in preschool children. J. Sports Sci. 2018, 36, 2289–2295. [Google Scholar] [CrossRef]
- Henriksson, P.; Cadenas-Sanchez, C.; Leppänen, M.; Nyström, C.D.; Ortega, F.B.; Pomeroy, J.; Ruiz, J.; Löf, M. Association of fat mass and fat-free mass with physical fitness in 4-year-old children: Results of MINISTOP trial. Nutrients 2016, 8, 473. [Google Scholar] [CrossRef]
- Matarma, T.; Lagström, H.; Hurme, S.; Tammelin, T.; Kulmala, J.; Barnett, L.M.; Koski, P. Motor skills in association with physical activity, sedentary time, body fat, and day care attendance in 5-6-year-old children–the STEPS Study. Scand. J. Med. Sci. Sports 2018, 28, 2668–2676. [Google Scholar] [CrossRef]
- Niederer, I.; Kriemler, S.; Zahner, L.; Bürgi, F.; Ebenegger, V.; Marques-Vidal, P.; Puder, J.J. BMI group-related differences in physical fitness and physical activity in preschool children: A cross-sectional analysis. Res. Q. Exerc. Sport 2012, 83, 12–19. [Google Scholar] [CrossRef]
- Gomes, T.N.; Katzmarzyk, P.T.; dos Santos, F.K.; Souza, M.; Pereira, S.; Maia, J.A.R. Overweight and obesity in Portuguese children: Prevalence and correlates. Int. J. Environ. Res. Public Health 2014, 11, 1398–1417. [Google Scholar] [CrossRef]
- Eriksson, M.; Lingfors, B.; Golsater, M. Trends in prevalence of thinness, overweight and obesity among Swedish children and adolescents between 2004 and 2015. Acta Paediatr. 2018, 107, 1818–1825. [Google Scholar] [CrossRef]
- Ball, G.D.C.; Savu, A.; Kaul, P. Changes in the prevalence of overweight, obesity, and severe obesity between 2010 and 2017 in preschoolers: A population-based study. Pediatr. Obes. 2019, 14, e12561. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, P.; Pařízková, J.; Procházková, L.; Cvrčková, L.; Dvořáková, H. Secular changes of adiposity in Czech children aged from 3 to 6 years: Latent obesity in preschool age. BioMed. Res. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.A.; Hernandez Alava, M.; Kelly, M.P.; Campbell, M.J. Family lifestyle dynamic and childhood obesity: Evidence from the milenium cohort study. BMC Public Health 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Kakebeeke, T.H.; Lanzi, S.; Zysset, A.E.; Arhab, A.; Messerli-Bürgy, N.; Stuelb, K.; Leeger-Aschmann, C.S.; Schmutz, E.A.; Meyer, A.H.; Kriemler, S.; et al. Association between body composition and motor performance in preschool children. Obes. Facts 2017, 10, 420–431. [Google Scholar] [CrossRef]
- Bláha, P. Anthropometry of Czech Preschool Children Aged 3–7 Years; Institute of Sport Medicine: Prague, Czechia, 1990. [Google Scholar]
- Eston, R.; Hawes, M.; Martin, A.; Reilly, T. Human body composition. In Kinanthropometry and Exercise Physiology Laboratory Manual. Volume 1: Anthropometry, 3rd. ed.; Eston, R., Reilly, T., Eds.; Taylor and Francis Group: London, UK; New York, NY, USA, 2009; pp. 3–53. ISBN 0415437229, 9780415437226. [Google Scholar]
- Matiegka, J. The testing of physical efficiency. Am. J. Phys. Anthropol. 1921, 4, 223–230. [Google Scholar] [CrossRef]
- Varekova, R.; Vareka, I. How to estimate overweight in pubescent asthmatics? Adv. Med. Sci. 2013, 58, 331–337. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Bláha, P.; Lisá, L.; Zamrazilová, H.; Brabec, M.; Kouba, M.; Vacková, B. Assessing of obese children body composition. Comparing clasis anthropometry methods with modern screening method—dual X-ray absorptiometry. Czech Slovak. Pediatr. 2004, 59, 176–181. [Google Scholar]
- Vignerová, J.; Brabec, M.; Bláha, P. Two centuries of growth among Czech children and youth. Econ. Hum. Biol. 2006, 4, 237–252. [Google Scholar] [CrossRef]
- Cardoso, H.F.; Caninas, M. Secular trends in social class differences of height, weight and BMI of boys from two schools in Lisbon, Portugal (1910–2000). Econ. Hum. Biol. 2010, 8, 111–120. [Google Scholar] [CrossRef]
- Kolodzyej, H.; Lopuszanska, M.; Lipovicz, A.; Szklarska, A.; Bielicki, T. Secular trends in body height and body mass in 19-year-old Polish men based on six national surveys from 1965 to 2010. Am. Hum. Biol. 2015, 27, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, N.Y.; Shin, H.Y.; Kim, J.H.; Moon, J.S.; Lee, C.G. Change in the height of Korean children and adolescents: Analysis from the Korea National Health and Nutrition Survey II and V. Korean J. Pediatr. 2015, 58, 336–340. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bodszar, E.; Zsakai, A.; Mascie-Taylor, N. Secular growth and maturation changes in Hungary in relation to socioeconomic and demographic changes. J. Biosoc. Sci. 2016, 48, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Topcu, S.; Simsek Orhon, F.; Ulukol, B.; Baskan, S. Secular trends in height, weight and body mass index of primary school children in Turkey berween 1993 and 2016. J. Pediatr. Endocrinol. Metab. 2017, 30, 1177–1186. [Google Scholar] [CrossRef]
- Kryst, L.; Woronkowicz, A.; Kowal, M.; Pilecki, M.W.; Sobiecki, J. Abdominal obesity screening tools in the aspects of secular trend. Anthropol. Anzeiger 2016, 73, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, E.; Somers, V.K.; Sochor, O.; Goel, K.; Lopez-Jimenez, F. The concept of normal weight obesity. Prog. Cardiovasc. Dis. 2014, 56, 426–433. [Google Scholar] [CrossRef]
- Marques-Vidal, P.; Bochud, M.; Mooser, V.; Paccaud, F.; Waeber, G.; Vollenweider, P. Prevalence of obesity and abdominal obesity in Lausanne population. BMC Public Helth 2008, 8. [Google Scholar] [CrossRef]
- Romero-Corral, A.; Somers, V.K.; Sierra-Johnson, J.; Korenfeld, Y.; Boarin, S.; Korinek, J.; Jensen, M.D.; Parati, G.; Lopez-Jimenez, F. Normal weight obesity: A risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur. Heart J. 2010, 31, 737–746. [Google Scholar] [CrossRef]
- Olafsdottir, A.S.; Torfadottir, J.E.; Arngrimsson, S.A. Health behavior and metabolic risk factors associated with normal weight obesity in adolescence. PLoS ONE 2016. [Google Scholar] [CrossRef]
- Musálek, M.; Pařízková, J.; Godina, E.; Bondareva, E.; Kokštejn, J.; Jírovec, J.; Vokounová, Š. Poor skeletal robustness on lower extremities and weak lean mass development on upper arm and calf: Normal weight obesity in middle-school-aged children (9 to 12). Front. Pediatr. 2018, 6. [Google Scholar] [CrossRef]
- Sakai, T.; Demura, S.; Fujii, K. Relationship between body composition and BMI in preschool children. Sport Sci. Health 2012, 7, 5–12. [Google Scholar] [CrossRef]
- Krombholz, A. Motor and cognitive performance of overweight preschool children. Percept. Mot. Skills 2013, 116, 40–57. [Google Scholar] [CrossRef] [PubMed]
- D’Hondt, E.; Deforche, B.; Gentier, I.; Verstuyf, J.; Vaeyens, R.; De Bourdeaudhuij, I.; Philippaerts, R.M.; Lenoir, M. A longitudinal study of gross motor coordination and weight status in children. Obesity 2014, 22, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Rietsch, K.; Godina, E.; Scheffler, C. Decreased External Skeletal Robustness in Schoolchildren—A Global Trend? Ten year comparison of Russian and German data. PLoS ONE 2013. [Google Scholar] [CrossRef]
- Rietsch, K.; Eccard, J.A.; Scheffler, C. Decreased external skeletal robustness due to reduced physical activity? Am. J. Hum. Biol. 2013, 25, 404–410. [Google Scholar] [CrossRef]
- Logan, S.W.; Scrabis-Fletcher, K.; Modleski, C.; Gatchell, N. The relationship between motor skill proficiency and body mass index in preschool children. Res. Q. Exerc. Sport 2011, 82, 442–448. [Google Scholar] [CrossRef]
- Vameghi, R.; Shams, A.; Shamsipour Dehkordi, P.S. The effect of age, sex and obesity on fundamental motor skills among 4 to 6 years-old children. Pak. J. Med Sci. 2013, 29, 586–589. [Google Scholar] [CrossRef]
- Olesen, L.G.; Kristensen, P.L.; Ried-Larsen, M.; Grontve, A.; Froberg, K. Physical activity and motor skills in children attending 43 preschools: A cross-sectional study. BMC Pediatr. 2014, 14. [Google Scholar] [CrossRef]
- Roberts, D.; Veneri, D.; Decker, R.; Ganotti, M. Weight status and gross motor skill in kindergarten children. Pediatr. Phys. Ther. 2012, 24, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Ruiz, M.; Estay Carvajal, J.; Calzadilla Nuñez, A.; Durán Aguero, S.; Díaz-Narváez, V.P. Comparison of psychomotor development in preschool Chilean normal weight versus overweight/obesity. Nutr. Hosp. 2015, 32, 151–155. [Google Scholar] [CrossRef]
- Vignerová, J.; Bláha, P.; Ošancová, K.; Roth, Z. Social inequality and obesity in Czech school children. Econ. Hum. Biol. 2004, 2, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.; Ng, B.; Sommer, M.; Heymsfield, S.B. Body composition by DXA. Bone 2017, 104, 101–105. [Google Scholar] [CrossRef] [PubMed]
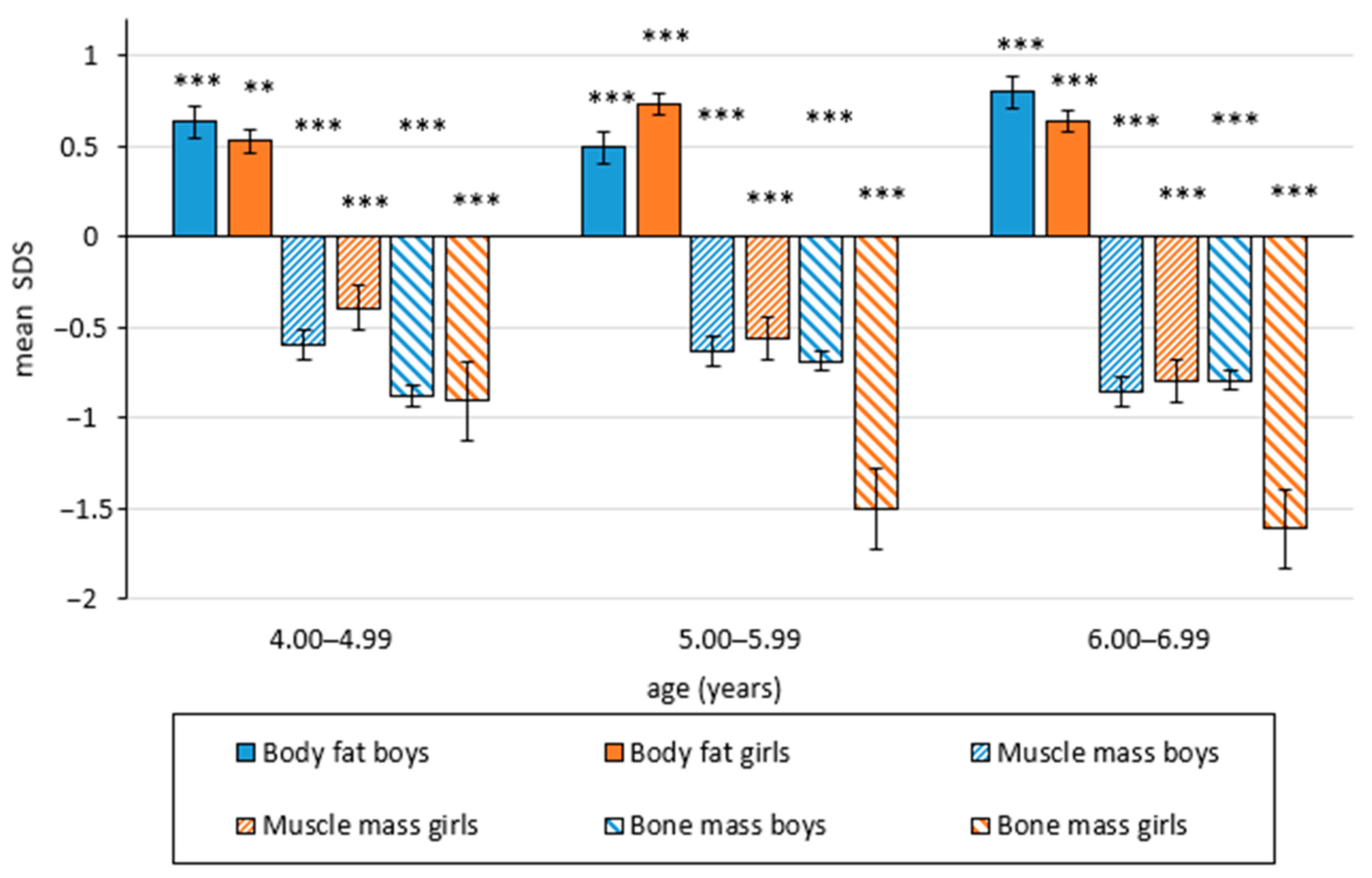
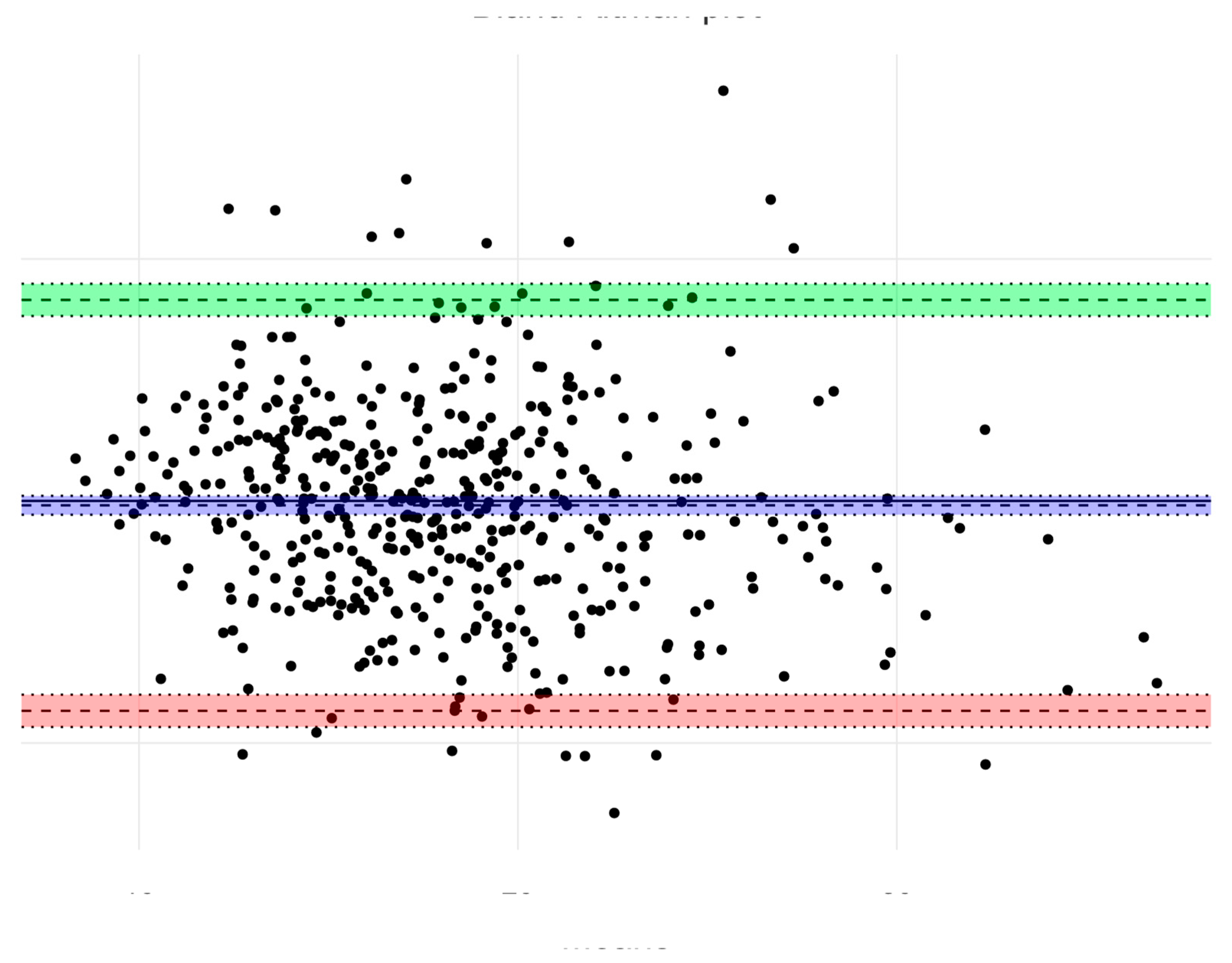
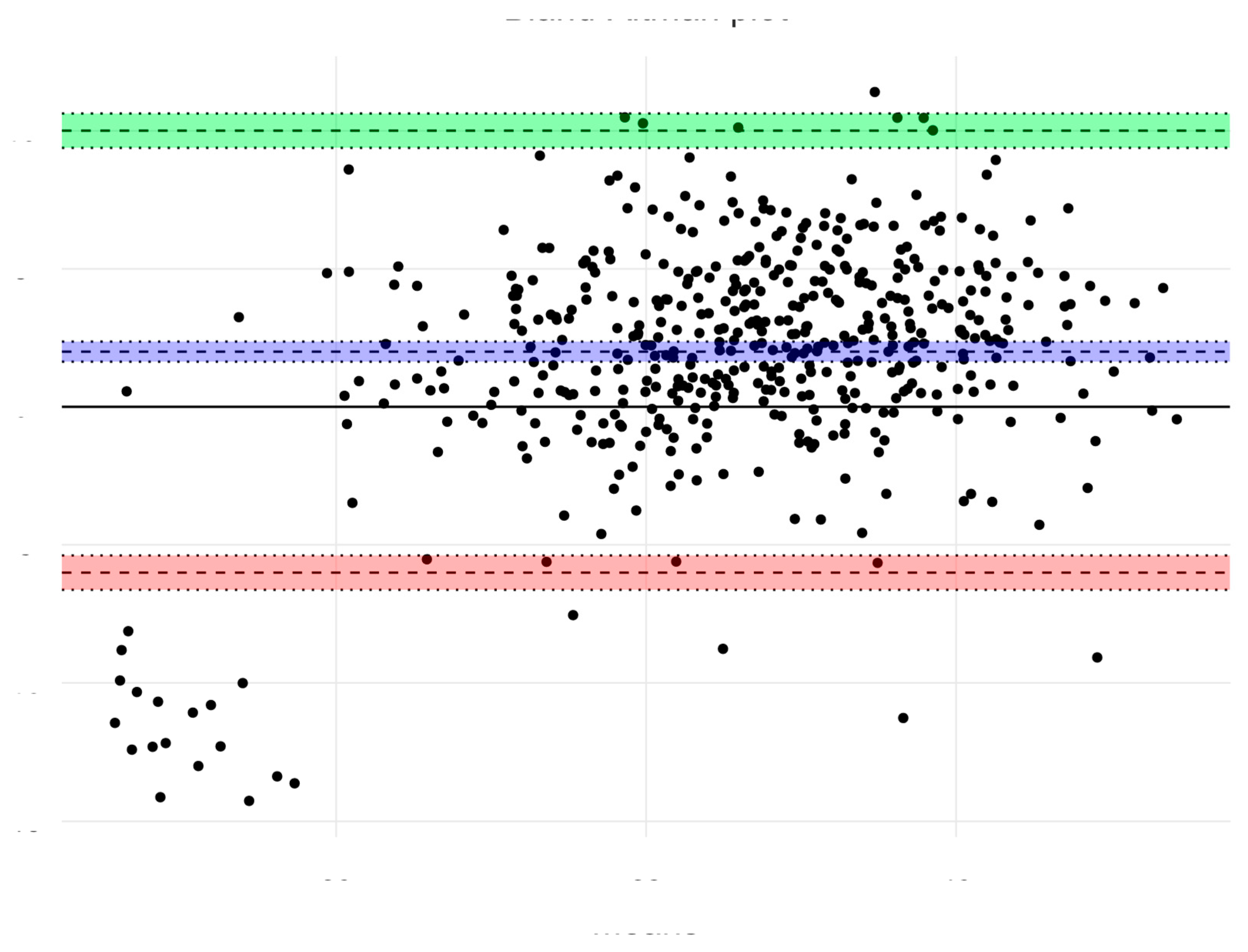
| Age (Years) | 4 | 5 | 6 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | A (n = 115) | B (n = 322) | A (n = 140) | B (n = 369) | A (n = 131) | B (n = 220) | ||||||
| Mean (SD) | Mean (SD) | p Values | Cohen’s d | Mean (SD) | Mean (SD) | p Values | Cohen’s d | Mean (SD) | Mean (SD) | p Values | Cohen’s d | |
| Body height (cm) | 107.6 (5.13) | 107.4 (4.79) | 0.582 | 0.0 | 115.0 (5.07) | 114.1 (5.01) | 0.164 | 0.2 | 120.3 (5.24) | 120.1 (4.86) | 0.626 | 0.1 |
| BMI (kgm−2) | 15.3 (1.25) | 15.5 (1.24) | 0.896 | 0.0 | 15.2 (1.20) | 15.5 (1.30) | 0.023 | 0.2 | 15.3 (1.29) | 15.6 (1.46) | 0.095 | 0.2 |
| Body fat (%) *) | 18.3 (4.02) | 16.1 (4.18) | <0.001 | 0.5 | 17.5 (5.58) | 15.6 (4.63) | <0.001 | 0.4 | 17.8 (6.50) | 15.0 (5.10) | <0.001 | 0.5 |
| Muscle mass (%) *) | 34.2 (3.19) | 36.5 (3.72) | <0.001 | 0.6 | 35.6 (3.21) | 37.5 (3.31) | <0.001 | 0.6 | 36.4 (3.16) | 39.2 (3.52) | <0.001 | 0.8 |
| Bone mass (%) *) | 19.1 (1.48) | 20.3 (1.43) | <0.001 | 0.9 | 19.5 (2.00) | 20.6 (1.65) | <0.001 | 0.7 | 19.6 (2.08) | 21.0 (2.62) | <0.001 | 0.5 |
| Age (Years) | 4 | 5 | 6 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | A (n = 112) | B (n = 314) | A (n = 153) | B (n = 391) | A (n = 107) | B (n = 191) | ||||||
| Mean (SD) | Mean (SD) | p Values | Cohen’s d | Mean (SD) | Mean (SD) | p Values | Cohen’sd | Mean (SD) | Mean (SD) | p Values | Cohen’s d | |
| Body height (cm) | 106.6 (5.16) | 106.8 (4.68) | 0.776 | 0.0 | 114.2 (5.18) | 113.6 (4.78) | 0.744 | 0.1 | 119.1 (5.40) | 119.5 (5.23) | 0.665 | 0.1 |
| BMI (kgm−2) | 15.5 (1.26) | 15.5 (1.37) | 0.793 | 0.0 | 15.5 (1.56) | 15.5 (1.63) | 0.987 | 0.0 | 15.4 (1.52) | 15.4 (1.58) | 0.622 | 0.0 |
| Body fat (%) *) | 20.9 (5.18) | 19.0 (4.98) | 0.002 | 0.4 | 22.2 (6.90) | 17.9 (5.22) | <0.001 | 0.8 | 20.9 (6.09) | 17.5 (5.49) | <0.001 | 0.6 |
| Muscle mass (%) *) | 34.3 (2.92) | 36.2 (3.97) | <0.001 | 0.7 | 35.3 (3.49) | 37.4 (3.80) | <0.001 | 0.7 | 35.9 (2.99) | 39.2 (4.16) | <0.001 | 0.8 |
| Bone mass (%) *) | 17.6 (1.67) | 19.1 (1.53) | <0.001 | 0.9 | 18.2 (1.55) | 19.1 (1.72) | <0.001 | 0.5 | 18.1 (1.75) | 19.8 (1.91) | <0.001 | 1.0 |
| Cut off Value | Boys (n = 337), Age 4–6 Years | Girls (n = 338), Age 4–6 Years | ||||
|---|---|---|---|---|---|---|
| BMI | % Fat | p Values | BMI | % Fat | p Values | |
| <10 percentile | 9.6 (6.5–12.8) | 10.9 (7.6–14.2) | 0.611 | 7.9 (5.0–10.8) | 7.9 (5.0–10.8) | 1.000 |
| 10–85 percentile | 80.7 (76.5–84.9) | 72.2 (67.4–77.0) | 0.011 * | 80.0 (75.5–84.1) | 73.5 (68.6–78.0) | 0.056 |
| >85 percentile | 9.7 (6.5–12.8) | 16.9 (12.9–20.9) | 0.009 ** | 12.1 (8.7–15.6) | 18.6 (14.5–22.8) | 0.025 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedlak, P.; Pařízková, J.; Samešová, D.; Musálek, M.; Dvořáková, H.; Novák, J. Secular Changes in Body Build and Body Composition in Czech Preschool Children in the Context of Latent Obesity. Children 2021, 8, 18. https://doi.org/10.3390/children8010018
Sedlak P, Pařízková J, Samešová D, Musálek M, Dvořáková H, Novák J. Secular Changes in Body Build and Body Composition in Czech Preschool Children in the Context of Latent Obesity. Children. 2021; 8(1):18. https://doi.org/10.3390/children8010018
Chicago/Turabian StyleSedlak, Petr, Jana Pařízková, Daniela Samešová, Martin Musálek, Hana Dvořáková, and Jan Novák. 2021. "Secular Changes in Body Build and Body Composition in Czech Preschool Children in the Context of Latent Obesity" Children 8, no. 1: 18. https://doi.org/10.3390/children8010018
APA StyleSedlak, P., Pařízková, J., Samešová, D., Musálek, M., Dvořáková, H., & Novák, J. (2021). Secular Changes in Body Build and Body Composition in Czech Preschool Children in the Context of Latent Obesity. Children, 8(1), 18. https://doi.org/10.3390/children8010018





