Management and Outcome of Traumatic Intracerebral Hemorrhage in 79 Infants and Children from a Single Level 1 Trauma Center
Abstract
:1. Introduction
2. Methods
2.1. Data Collection
2.2. Treatment Procedures
2.3. Data Analysis
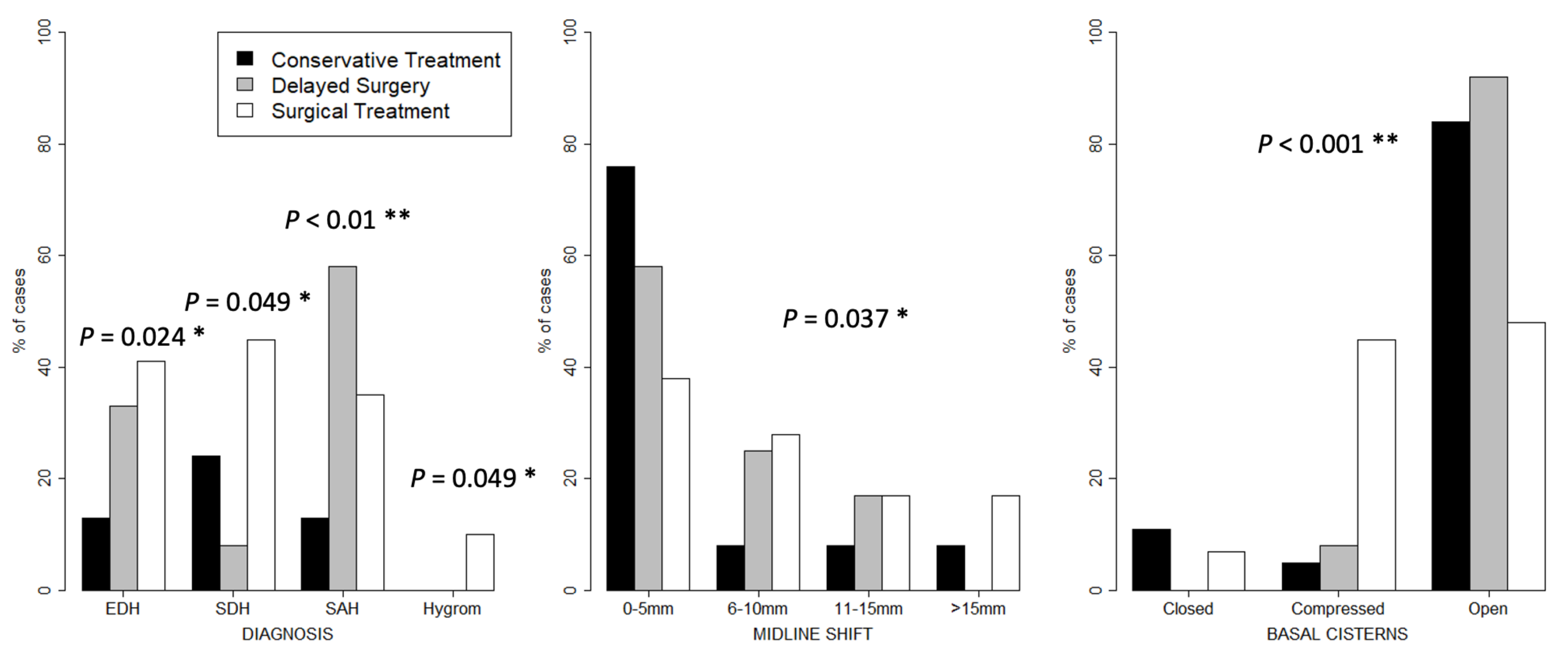
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIS | Abbreviated Injury Scale |
| CA | Child Abuse |
| CT | Computed Tomography |
| EDH | Epidural Hematoma |
| SDH | Subdural Hematoma |
| GCS | Glasgow Coma Scale |
| ICP | Intracranial Pressure |
| ICU | Intermediate Care Unit |
| ISS | Injury Severity Score |
| ICB | Intracerebral Bleeding |
| TBI | Traumatic Brain Injury |
STROBE Guidelines
| Item No | Recommendation | Please Insert Check Where Included or N/A Where Not Applicable | |
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | check |
| (b) Provide, in the abstract, an informative and balanced summary of what was performed and what was found | check | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | check |
| Objectives | 3 | State specific objectives, including any pre specified hypotheses | check |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | check |
| Setting | 5 | Describe the setting, locations and relevant dates, including periods of recruitment, treatment, follow up and data collection | check |
| Participants | 6 | (a) Cohort study—Provide the eligibility criteria and the sources and methods of selection of participants. Describe methods of follow up Case-control study—Provide the eligibility criteria and the sources and methods of case ascertainment and control selection. Provide the rationale for the choice of cases and controls Cross-sectional study—Provide the eligibility criteria and the sources and methods of selection of participants | check |
| (b) Cohort study—For matched studies, provide matching criteria and number of treated and untreated Case-control study—For matched studies, provide matching criteria and the number of controls per case | |||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders and effect modifiers. Provide diagnostic criteria, if applicable | check |
| Data sources/measurement | 8 * | For each variable of interest, provide sources of data and details of methods of assessment (measurement) Describe comparability of assessment methods if there is more than one group | check |
| Bias | 9 | Describe any efforts to address potential sources of bias | check |
| Study size | 10 | Explain how the study size was arrived at | check |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | check |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding factors | check |
| / | (b) Describe any methods used to examine subgroups and interactions | / | |
| / | (c) Explain how missing data were addressed | check | |
| / | (d) If applicable, explain how loss to follow up was addressed | check | |
| / | (e) Describe any sensitivity analyses | / | |
| Results | |||
| Participants | 13 * | (a) Report numbers of individuals at each stage of study—e.g., numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow up and analyzed | check |
| / | (b) Give reasons for nonparticipation at each stage | check | |
| Descriptive data | 14 * | (a) Give characteristics of study participants (e.g., demographic, clinical, social) and information on other treatments and potential confounders | check |
| / | (b) Indicate number of participants with missing data for each variable of interest | check | |
| / | (c) Cohort study—Summarize follow-up time (e.g., average and total amount) | check | |
| Outcome data | 15 * | Report numbers of outcome events or summary measures over time | N/A |
| Main results | 16 | (a) Provide unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g., 95% confidence interval). Make clear which confounders were adjusted for and why they were included | / |
| / | (b) Report category boundaries when continuous variables were categorized | N/A | |
| / | (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | N/A | |
| Other analyses | 17 | Report other analyses performed—e.g., analyses of subgroups and interactions and sensitivity analyses | N/A |
| Discussion | |||
| Key results | 18 | Summarise key results with reference to study objectives | check |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | check |
| Interpretation | 20 | Provide a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies and other relevant evidence | check |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | check |
| Other information | |||
| Funding | 22 | Provide the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | check |
| * Provide information separately for cases and controls. Note: An Explanation and Elaboration article discusses each checklist item and provides methodological background and published examples of transparent reporting. Information on the STROBE Initiative is available at http://www.strobe-statement.org, access date 15 January 2021. | |||
References
- Parslow, R.C.; Morris, K.P.; Tasker, R.; Forsyth, R.; Hawley, C.A. Epidemiology of traumatic brain injury in children receiving intensive care in the UK. Arch. Dis. Child. 2005, 90, 1182–1187. [Google Scholar] [CrossRef]
- Duhaime, A.C.; Alario, A.J.; Lewander, W.J.; Schut, L.; Sutton, L.N.; Seidl, T.S.; Nudelman, S.; Budenz, D.; Hertle, R.; Tsiaras, W. Head injury in very young children: Mechanisms, injury types, and ophthalmologic findings in 100 hospitalized patients younger than 2 years of age. Pediatrics 1992, 90, 179–185. [Google Scholar]
- Jordan, L.C.; Johnston, S.C.; Wu, Y.W.; Sidney, S.; Fullerton, H.J. The importance of cerebral aneurysms in childhood hemorrhagic stroke: A population-based study. Stroke 2009, 40, 400–405. [Google Scholar] [CrossRef] [Green Version]
- Adelson, P.D.; Bratton, S.L.; Carney, N.A.; Chesnut, M.R.; Du Courday, H.E.M.; Goldstein, B.; Kochanek, M.P.; Miller, C.H.; Partington, M.D.; Selden, N.R.; et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 1: Introduction. Pediatr. Crit. Care Med. 2003, 4 (Suppl. 3), S2–S4. [Google Scholar] [CrossRef] [PubMed]
- Mandera, M.; Zralek, C.; Krawczyk, I.; Życiński, A.; Wencel, T.; Bażowski, P. Surgery or conservative treatment in children with traumatic intracerebral haematoma. Child’s Nerv. Syst. 1999, 15, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Bullock, R.; Golek, J.; Blake, G. Traumatic intracerebral hematoma—Which patients should undergo surgical evacuation? CT scan features and ICP monitoring as a basis for decision making. Surg. Neurol. 1989, 32, 181–187. [Google Scholar] [CrossRef]
- Nordstrom, C.-H.; Messeter, K.; Sundbärg, J.G.; Wahlander, S. Severe traumatic brain lesions in Sweden. Part I: Aspects of management in non-neurosurgical clinics. Brain Inj. 1989, 3, 247–265. [Google Scholar] [CrossRef]
- Singounas, E.G. Severe head injury in a paediatric population. J. Neurosurg. Sci. 1992, 36, 201–206. [Google Scholar]
- Lo, W.D.; Lee, J.; Rusin, J.; Perkins, E.; Roach, E.S. Intracranial hemorrhage in children: An evolving spectrum. Arch Neurol. 2008, 65, 1629–1633. [Google Scholar] [CrossRef] [Green Version]
- Zakhary, M.M.; Wesolowski, J.R.; Sewick, A.E.; Carlson, M.; Mehrotha, N.; Malý, P.; Sundgren, P.C. Prevalence and Etiology of Intracranial Hemorrhage in Term Children Under the Age of Two Years: A Retrospective Study of Computerized Tomographic Imaging and Clinical Outcome in 798 Children. Acad. Radiol. 2009, 16, 572–577. [Google Scholar] [CrossRef]
- Hamilton, M.; Mrazik, M.; Johnson, D.W. Incidence of delayed intracranial hemorrhage in children after un-complicated minor head injuries. Pediatrics 2010, 126, e33–e39. [Google Scholar] [CrossRef] [PubMed]
- Karibe, H.; Kameyama, M.; Hayashi, T.; Narisawa, A.; Tominaga, T. Acute Subdural Hematoma in Infants with Abusive Head Trauma: A Literature Review. Neurol. Med. Chir. 2016, 56, 264–273. [Google Scholar] [CrossRef] [Green Version]
- Sorokina, E.G.; Semenova, Z.B.; Averianova, N.S.; Karaseva, O.V.; Arsenieva, E.; Luk’yanov, V.I.; Reutov, V.P.; Asanov, A.Y.; Roshal, L.M.; Pinelis, V.G. APOE gene polymorphism and markers of brain damage in the outcomes of severe traumatic brain injury in children. Zhurnal Nevrol. Psikhiatrii Korsakova 2020, 120, 72–80. [Google Scholar] [CrossRef]
- Ferrete-Araujo, A.M.; Egea-Guerrero, J.J.; Vilches-Arenas, A.; Godoy, D.A.; Murillo-Cabezas, F. Predictors of mortality and poor functional outcome in severe spontaneous intracerebral hemorrhage: A prospective observational study. Med. Intensiva 2015, 39, 422–432. [Google Scholar] [CrossRef]
- Jordan, L.C.; Kleinman, J.T.; Hillis, A.E. Intracerebral Hemorrhage Volume Predicts Poor Neurologic Outcome in Children. Stroke 2009, 40, 1666–1671. [Google Scholar] [CrossRef]
- Kleinman, J.T.; Hillis, A.E.; Jordan, L.C. ABC/2: Estimating intracerebral haemorrhage volume and total brain volume, and predicting outcome in children. Dev. Med. Child Neurol. 2010, 53, 281–284. [Google Scholar] [CrossRef] [Green Version]
- Kochanek, P.M.; Tasker, R.C.; Carney, N.; Totten, A.M.; Adelson, P.D.; Selden, N.R.; Davis-O’Reilly, C.; Hart, E.L.; Bell, M.J.; Bratton, S.L.; et al. Guidelines for the Management of Pediatric Severe Traumatic Brain Injury, Third Edition: Update of the Brain Trauma Foundation Guidelines, Executive Summary. Neurosurgery 2019, 84, 1169–1178. [Google Scholar] [CrossRef] [Green Version]
- Das Schädel-Hirn-Trauma im Kindesalter. 2018. Available online: http://www.leitliniensekretariat.de/files/MyLayout/pdf/024-018l_S2k_Schaedel-Hirn-Trauma_im_Kindesalter-2011-03.pdf (accessed on 20 September 2021).
- Maas, A.I.; Hukkelhoven, C.W.; Marshall, L.F.; Steyerberg, E.W. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: A comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery 2005, 57, 1173–1782. [Google Scholar] [CrossRef] [Green Version]
- Meyer-Heim, A.D.; Boltshauser, E. Spontaneous intracranial haemorrhage in children: Aetiology, presentation and outcome. Brain Dev. 2003, 25, 416–421. [Google Scholar] [CrossRef]
- Lin, C.L.; Loh, J.K.; Kwan, A.L.; Howng, S.L. Spontaneous intracerebral hemorrhage in children. Kaohsiung J. Med. Sci. 1999, 15, 146–151. [Google Scholar] [PubMed]
- Al-Jarallah, A.; Al-Rifai, M.T.; Riela, A.R.; Roach, E.S. Nontraumatic Brain Hemorrhage in Children: Etiology and Presentation. J. Child Neurol. 2000, 15, 284–289. [Google Scholar] [CrossRef]
- Kumar, R.; Shukla, D.; Mahapatra, A. Spontaneous Intracranial Hemorrhage in Children. Pediatr. Neurosurg. 2009, 45, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.K.; Park, C.K.; Kim, M.C.; Kim, D.S.; Song, J.U. Traumatic isolated intracerebral hemorrhage in children. Child’s Nerv. Syst. 1989, 5, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Ruf, B.; Heckmann, M.; Schroth, I.; Hügens-Penzel, M.; Reiss, I.; Borkhardt, A.; Gortner, L.; Jödicke, A. Early decompressive craniectomy and duraplasty for refractory intracranial hypertension in children: Results of a pilot study. Crit. Care 2003, 7, R133–R138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mhanna, M.J.; Mallah, W.E.; Verrees, M.; Shah, R.; Super, D.M. Outcome of children with severe traumatic brain injury who are treated with decompressive craniectomy. J. Neurosurg. Pediatr. 2015, 16, 508–514. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.; Butt, W.; Rosenfeld, J.; Shann, F.; Ditchfield, M.; Lewis, E.; Klug, G.; Wallace, D.; Henning, R.; Tibballs, J. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Child’s Nerv. Syst. 2001, 17, 154–162. [Google Scholar] [CrossRef]
- Skoglund, T.S.; Eriksson-Ritzén, C.; Jensen, C.; Rydenhag, B. Aspects on Decompressive Craniectomy in Patients with Traumatic Head Injuries. J. Neurotrauma 2006, 23, 1502–1509. [Google Scholar] [CrossRef]
- Thomale, U.-W.; Graetz, D.; Vajkoczy, P.; Sarrafzadeh, A.S. Severe traumatic brain injury in children—A single center experience regarding therapy and long-term outcome. Child’s Nerv. Syst. 2010, 26, 1563–1573. [Google Scholar] [CrossRef]
- Sorokina, E.G.; Reutov, V.P.; Pinelis, V.; Vinskaya, N.P.; Vergun, O.V.; Khodorov, B.I. The mechanism of potentiation of the glutamate-induced neurotoxicity by serum albumin. A possible role of nitric oxide. Membr. Cell Biol. 2000, 13, 389–396. [Google Scholar]
- Sorokina, E.G.; Semenova, Z.B.; Reutov, V.P.; Arsenieva, E.N.; Karaseva, O.V.; Fisenko, A.P.; Roshal, L.M.; Pinelis, V.G. Brain Biomarkers in Children After Mild and Severe Traumatic Brain Injury; Springer Science and Business Media LLC.: Berlin/Heidelberg, Germany, 2021; Volume 131, pp. 103–107. [Google Scholar]
- Wolf, H.; Machold, W.; Frantal, S.; Kecht, M.; Pajenda, G.; Leitgeb, J.; Widhalm, H.; Hajdu, S.; Sarahrudi, K. Risk factors indicating the need for cranial CT scans in elderly patients with head trauma: An Austrian trial and comparison with the Canadian CT Head Rule. J. Neurosurg. 2014, 120, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Manfiotto, M.; Beccaria, K.; Rolland, A.; Paternoster, G.; Plas, B.; Boetto, S.; Vinchon, M.; Mottolese, C.; Beuriat, P.-A.; Szathmari, A.; et al. Decompressive Craniectomy in Children with Severe Traumatic Brain Injury: A Multicenter Retrospective Study and Literature Review. World Neurosurg. 2019, 129, e56–e62. [Google Scholar] [CrossRef] [PubMed]
- Beuriat, P.-A.; Javouhey, E.; Szathmari, A.; Courtil-Tesseydre, S.; Desgranges, F.P.; Grassiot, B.; Hequet, O.; Mottolese, C. Decompressive craniectomy in the treatment of post-traumatic intracranial hypertension in children: Our philosophy and indications. J. Neurosurg. Sci. 2015, 59, 405–428. [Google Scholar] [PubMed]
- Ghajar, J.; Hariri, R.J. Management of Pediatric Head Injury. Pediatr. Clin. N. Am. 1992, 39, 1093–1125. [Google Scholar] [CrossRef]
- Caroli, M.; Locatelli, M.; Campanella, R.; Balbi, S.; Martinelli, F.; Arienta, C. Multiple intracranial lesions in head injury: Clinical considerations, prognostic factors, management, and results in 95 patients. Surg. Neurol. 2001, 56, 82–88. [Google Scholar] [CrossRef]
- Lobato, R.D.; Cordobes, F.; Rivas, J.J.; De La Fuente, M.; Montero, A.; Barcena, A.; Perez, C.; Cabrera, A.; Lamas, E. Outcome from severe head injury related to the type of intracranial lesion. J. Neurosurg. 1983, 59, 762–774. [Google Scholar] [CrossRef]
- Bullock, M.R.; Chesnut, R.; Ghajar, J.; Gordon, D.; Härtl, R.; Newell, D.W.; Servadei, F.; Walters, B.C.; Wilberger, J. Surgical Management of Traumatic Parenchymal Lesions. Neurosurgery 2006, 58, S2–S25. [Google Scholar] [CrossRef]
- Lackland, D.T.; Roccella, E.J.; Deutsch, A.F.; Fornage, M.; George, M.G.; Howard, G.; Kissela, B.M.; Kittner, S.J.; Lichtman, J.H.; Lisabeth, L.D.; et al. Factors influencing the decline in stroke mortality: A statement from the American Heart Association/American Stroke Association. Stroke 2014, 45, 315–353. [Google Scholar] [CrossRef] [Green Version]
- Zahuranec, D.B.; Lisabeth, L.D.; Sánchez, B.N.; Smith, M.A.; Brown, D.L.; Garcia, N.M.; Skolarus, L.E.; Meurer, W.J.; Burke, J.F.; Adelman, E.E.; et al. Intracerebral hemorrhage mortality is not changing despite declining incidence. Neurology 2014, 82, 2180–2186. [Google Scholar] [CrossRef] [Green Version]
- Alberico, A.M.; Ward, J.D.; Choi, S.C.; Marmarou, A.; Young, H.F. Outcome after severe head injury. Relationship to mass lesions, diffuse injury, and ICP course in pediatric and adult patients. J. Neurosurg. 1987, 67, 648–656. [Google Scholar] [CrossRef]
- Wu, E.; Marthi, S.; Asaad, W.F. Predictors of Mortality in Traumatic Intracranial Hemorrhage: A National Trauma Data Bank Study. Front. Neurol. 2020, 11, 587587. [Google Scholar] [CrossRef]
- Figaji, A.A.; Fieggen, A.G.; Peter, J.C.; Fieggen, G. Early decompressive craniotomy in children with severe traumatic brain injury. Child’s Nerv. Syst. 2003, 19, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Reece, R.M.; Sege, R. Childhood head injuries: Accidental or inflicted? Arch. Pediatr. Adolesc. Med. 2000, 154, 11–15. [Google Scholar] [PubMed]
- Vinchon, M.; Defoort-Dhellemmes, S.; Desurmont, M.; Dhellemmes, P. Accidental and nonaccidental head injuries in infants: A prospective study. J. Neurosurg. Pediatr. 2005, 102, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Duhaime, A.C.; Christian, C.W.; Rorke, L.B.; Zimmerman, R.A. Nonaccidental head injury in infants—The “shaken-baby syndrome”. N. Engl. J. Med. 1998, 338, 1822–1829. [Google Scholar] [CrossRef]
- Parent, A.D. Pediatric chronic subdural hematoma: A retrospective comparative analysis. Pediatr. Neurosurg. 1992, 18, 266–271. [Google Scholar] [CrossRef]
- Case, M.E. Inflicted Traumatic Brain Injury in Infants and Young Children. Brain Pathol. 2008, 18, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Jayawant, S.; Parr, J. Outcome following subdural haemorrhages in infancy. Arch. Dis. Child. 2007, 92, 343–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Measure | Conservative | Delayed Surgery | Operative | Total | p-Value |
|---|---|---|---|---|---|
| Total N (%) | 38 (48%) | 12 (15%) | 29 (37%) | 79 | |
| Age (median, IQR) | 8.5 (6–13) | 7.5 (2.75–11.75) | 10 (2–14) | 8 (3.5–14) | 0.874 |
| Gender (N, % male) | 21 (55%) | 7 (58%) | 19 (66%) | 47 (60%) | 0.695 |
| Trauma mechanism (N, %) | / | / | / | / | / |
| Brawl | 0 | 0 | 1 (3%) | 1 (1%) | 0.629 |
| Fall (50–150 cm) | 2 (5%) | 1 (8%) | 1 (3%) | 4 (5%) | |
| Fall (<50 cm) | 2 (5%) | 0 | 0 | 2 (3%) | |
| Fall (>150 cm) | 6 (16%) | 4 (33%) | 11 (38%) | 21 (26%) | / |
| Traffic accident | 23 (61%) | 7 (58%) | 15 (52%) | 45 (57%) | / |
| Sports | 2 (5%) | 0 | 1 (3%) | 3 (4%) | |
| Battered child syndrome | 2 (5%) | 0 | 0 | 2 (3%) | |
| Other | 1 (3%) | 0 | 0 | 1 (1%) | |
| Multiple trauma (N, % Yes) | 24 (63%) | 9 (75%) | 26 (90%) | 59 (75%) | 0.04 * |
| Measure/Treatment Type | Conservative | Delayed Surgery | Operative | Total | p-Value |
|---|---|---|---|---|---|
| Total N (%) | 38 (48%) | 12 (15%) | 29 (37%) | 79 | |
| Symptoms indicating TBI (N, % Yes) | / | / | / | / | / |
| External Swellings | 19 (50%) | 8 (67%) | 12 (41%) | 39 (49%) | 0.335 |
| Nausea | 6 (16%) | 2 (17%) | 1 (3%) | 9 (11%) | <0.01 |
| Vomiting | 5 (13%) | 3 (25%) | 4 (14%) | 12 (15%) | 0.41 |
| Unconsciousness (N, % present) | 17 (45%) | 7 (58%) | 21 (72%) | 45 (57%) | 0.22 |
| Neurological status (N, %) | / | / | / | / | / |
| Normal | 15 (40%) | 0 | 1 (3%) | 16 (20%) | <0.001 ** |
| Somnolent | 12 (32%) | 5 (42%) | 5 (17%) | 22 (28%) | |
| Comatose | 11 (29%) | 6 (50%) | 23 (79%) | 40 (51%) | |
| Pupils (N, %) | / | / | / | / | / |
| Both reactive | 28 (74%) | 8 (67%) | 17 (59%) | 53 (67%) | 0.679 |
| One reactive | 4 (11%) | 1 (8%) | 3 (10%) | 8 (10%) | |
| None reactive | 6 (16%) | 3 (25%) | 9 (31%) | 18 (23%) | |
| ISS (median, IQR) | 16 (9–21) | 25 (17–30) | 24 (17–29) | 20 (15–26) | <0.01 ** |
| First pediatric GCS (median, IQR) | 8.5 (6–13) | 7.5 (3–12) | 10 (2–14) | 8 (3.5–14) | 0.869 |
| Admission pediatric GCS (median, IQR) | 8.5 (6–13) | 7.5 (3–12) | 10 (2–14) | 8 (3.5–14) | 0.869 |
| Vertebral Fracture (N, %) | / | / | / | / | / |
| Neck Region | 4 (11%) | 1 (8%) | 1 (3%) | 6 (8%) | 0.553 |
| Thorax Region | 2 (5%) | 0 | 1 (3%) | 3 (4%) | 0.7 |
| Additional Injuries (N, %) | / | / | / | / | / |
| Upper extremity Fracture | 6 (16%) | 2 (17%) | 3 (10%) | 11 (14%) | 0.779 |
| Lower Extremity Fracture | 5 (13%) | 2 (17%) | 5 (17%) | 12 (15%) | 0.824 |
| Injury to thoracic region | 8 (21%) | 4 (33%) | 11 (38%) | 23 (29%) | 0.18 |
| Injury to abdominal region | 5 (13%) | 2 (17%) | 5 (17%) | 12 (15%) | 0.888 |
| Rotterdam CT Score (N, %) | / | / | / | / | / |
| 1 | 0 | 0 | 0 | 0 | 0.038 * |
| 2 | 6 (16%) | 3 (25%) | 3 (10%) | 12 (15%) | |
| 3 | 24 (63%) | 7 (58%) | 9 (31%) | 40 (51%) | |
| 4 | 2 (5%) | 2 (17%) | 8 (28%) | 12 (15%) | |
| 5 | 3 (8%) | 0 | 7 (24%) | 10 (13%) | |
| 6 | 3 (8%) | 0 | 2 (7%) | 5 (6%) |
| Measure/Treatment Type | Conservative | Delayed Surgery | Operative | Total | p-Value |
|---|---|---|---|---|---|
| Total N (%) | 38 (48%) | 12 (15%) | 29 (37%) | 79 | |
| Transport (N, % Air) | 11 (29%) | 5 (42%) | 17 (59%) | 33 (42%) | 0.142 |
| Intubation (N, %) | 12 (32%) | 7 (58%) | 23 (79%) | 42 (53%) | <0.001 ** |
| X-Ray performed (N, %) | 27 (71%) | 10 (83%) | 25 (86%) | 62 (79%) | 0.296 |
| CT scan performed (N, %) | 38 (100%) | 12 (100%) | 29 (100%) | 79 (100%) | 1 |
| MR performed (N, %) | 7 (18%) | 3 (25%) | 7 (24%) | 17 (22%) | 0.811 |
| ICU days (N, %) | / | / | / | / | / |
| No ICU admission | 22 (58%) | 0 | 0 | 22 (28%) | <0.001 ** |
| ≤10 days | 11 (29%) | 4 (33%) | 12 (41%) | 27 (34%) | |
| 11–20 days | 1 (3%) | 3 (25%) | 5 (17%) | 9 (11%) | |
| 21–30 days | 3 (8%) | 3 (25%) | 6 (21%) | 12 (15%) | |
| Over 30 days | 2 (5%) | 1 (8%) | 6 (21%) | 9 (11%) |
| Measure | Delayed Surgery | Operative | Total | p-Value |
|---|---|---|---|---|
| / | 12 (29%) | 29 (71%) | 41 | |
| Surgery time (N, %) | / | / | / | / |
| <1 h | 0 | 23 (100%) | 23 (76%) | <0.001 ** |
| <24 h | 12 (100%) | 0 | 12 (29%) | |
| <1 week | 1 (8%) | 0 | 1 (2%) | |
| <2 weeks | 1 (8%) | 0 | 1 (2%) | |
| Delayed after 4 h | 2 (17%) | 0 | 2 (5%) | |
| TBI surgeries within 24 h (N, %) | / | / | / | / |
| One | 9 (75%) | 28 (97%) | 37 (90%) | 0.067 |
| Two | 1 (8%) | 1 (3%) | 2 (5%) | |
| None | 2 (17%) | 0 | 2 (5%) | |
| Overall number of TBI surgeries (N, %) | / | / | / | / |
| One | 9 (75%) | 19 (66%) | 28 (68%) | 0.9 |
| Two | 2 (17%) | 7 (24%) | 9 (22%) | |
| Three | 1 (8%) | 2 (7%) | 3 (7%) | |
| Four | 0 | 1 (3%) | 1 (2%) | |
| Other Surgery (N, %) | / | / | / | / |
| Multiple surgery | 0 | 3 (10%) | 3 (7%) | 0.792 |
| Osteosynthesis (external) | 2 (17%) | 2 (7%) | 4 (10%) | |
| Osteosynthesis (internal) | 1 (8%) | 2 (7%) | 3 (7%) | |
| Mediastinal drain | 0 | 1 (3%) | 1 (2%) | |
| Other | 1 (8%) | 2 (7%) | 3 (7%) | |
| None | 8 (67%) | 18 (62%) | 26 (63%) | |
| Parenchymal ICP monitor (N, %) | 9 (75%) | 25 (86%) | 34 (83%) | 0.397 |
| Ventricular drain (N, %) | 5 (42%) | 4 (14%) | 9 (22%) | 0.092 |
| Days of ICP monitoring (N, %) | / | / | / | / |
| ≤10 days | 5 (41%) | 16 (55%) | 21 (51%) | 0.323 |
| 11–20 days | 2 (17%) | 4 (14%) | 7 (17%) | |
| 21–30 days | 2 (17%) | 4 (14%) | 6 (15%) | |
| 31+ days | 1 (8%) | 2 (7%) | 3 (7%) | |
| None | 2 (17%) | 3 (10%) | 5 (12%) |
| Measure/Treatment Type | Conservative | Delayed Surgery | Operative | Total |
|---|---|---|---|---|
| 38 (48%) | 12 (15%) | 29 (37%) | 79 | |
| GOS–hospital discharge (N, %) | / | / | / | / |
| Death | 4 (11%) | 1 (8%) | 6 (21%) | 11 (14%) |
| Vegetative state | 0 | 0 | 1 (3%) | 1 (3%) |
| Severe disability | 2 (5%) | 3 (25%) | 3 (10%) | 8 (10%) |
| Moderate disability | 3 (8%) | 2 (17%) | 12 (41%) | 17 (22%) |
| Good recovery | 28 (74%) | 6 (50%) | 6 (21%) | 40 (51%) |
| Unknown | 1 (3%) | 0 | 1 (3%) | 2 (3%) |
| GOS–follow up (N, %) | / | / | / | / |
| Death | 4 (11%) | 1 (8%) | 6 (21%) | 11 (14%) |
| Vegetative state | 0 | 0 | 1 (3%) | 1 (1%) |
| Severe disability | 0 | 1 (8%) | 1 (3%) | 2 (3%) |
| Moderate disability | 1 (3%) | 1 (8%) | 3 (10%) | 5 (6%) |
| Good recovery | 22 (58%) | 6 (50%) | 13 (45%) | 41 (52%) |
| Unknown | 11 (29%) | 3 (25%) | 5 (17%) | 19 (24%) |
| Time of Death (N, %) | / | / | / | / |
| Survivors | 34 (90%) | 11 (92%) | 23 (79%) | 68 (86%) |
| Within 24 h | 3 (8%) | 0 | 3 (10%) | 6 (8%) |
| Within 48 h | 1 (3%) | 0 | 2 (7%) | 3 (4%) |
| Within 14 days | 0 | 1 (8%) | 0 | 1 (1%) |
| Within 21 days | 0 | 0 | 1 (3%) | 1 (1%) |
| Cause of death (N, %) | / | / | / | / |
| Survivors | 34 (90%) | 11 (92%) | 23 (79%) | 68 (86%) |
| Brain death | 2 (5%) | 0 | 4 (14%) | 6 (8%) |
| Cardiovascular failure | 2 (5%) | 1 (8%) | 0 | 2 (4%) |
| Respiratory/Pulmonary failure | 0 | 0 | 1 (3%) | 1 (1%) |
| Multiorgan failure (sepsis) | 0 | 0 | 1 (3%) | 1 (1%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binder, H.; Majdan, M.; Leitgeb, J.; Payr, S.; Breuer, R.; Hajdu, S.; Tiefenboeck, T.M. Management and Outcome of Traumatic Intracerebral Hemorrhage in 79 Infants and Children from a Single Level 1 Trauma Center. Children 2021, 8, 854. https://doi.org/10.3390/children8100854
Binder H, Majdan M, Leitgeb J, Payr S, Breuer R, Hajdu S, Tiefenboeck TM. Management and Outcome of Traumatic Intracerebral Hemorrhage in 79 Infants and Children from a Single Level 1 Trauma Center. Children. 2021; 8(10):854. https://doi.org/10.3390/children8100854
Chicago/Turabian StyleBinder, Harald, Marek Majdan, Johannes Leitgeb, Stephan Payr, Robert Breuer, Stefan Hajdu, and Thomas M. Tiefenboeck. 2021. "Management and Outcome of Traumatic Intracerebral Hemorrhage in 79 Infants and Children from a Single Level 1 Trauma Center" Children 8, no. 10: 854. https://doi.org/10.3390/children8100854
APA StyleBinder, H., Majdan, M., Leitgeb, J., Payr, S., Breuer, R., Hajdu, S., & Tiefenboeck, T. M. (2021). Management and Outcome of Traumatic Intracerebral Hemorrhage in 79 Infants and Children from a Single Level 1 Trauma Center. Children, 8(10), 854. https://doi.org/10.3390/children8100854








