The Intertemporal Role of Respiratory Support in Improving Neonatal Outcomes: A Narrative Review
Abstract
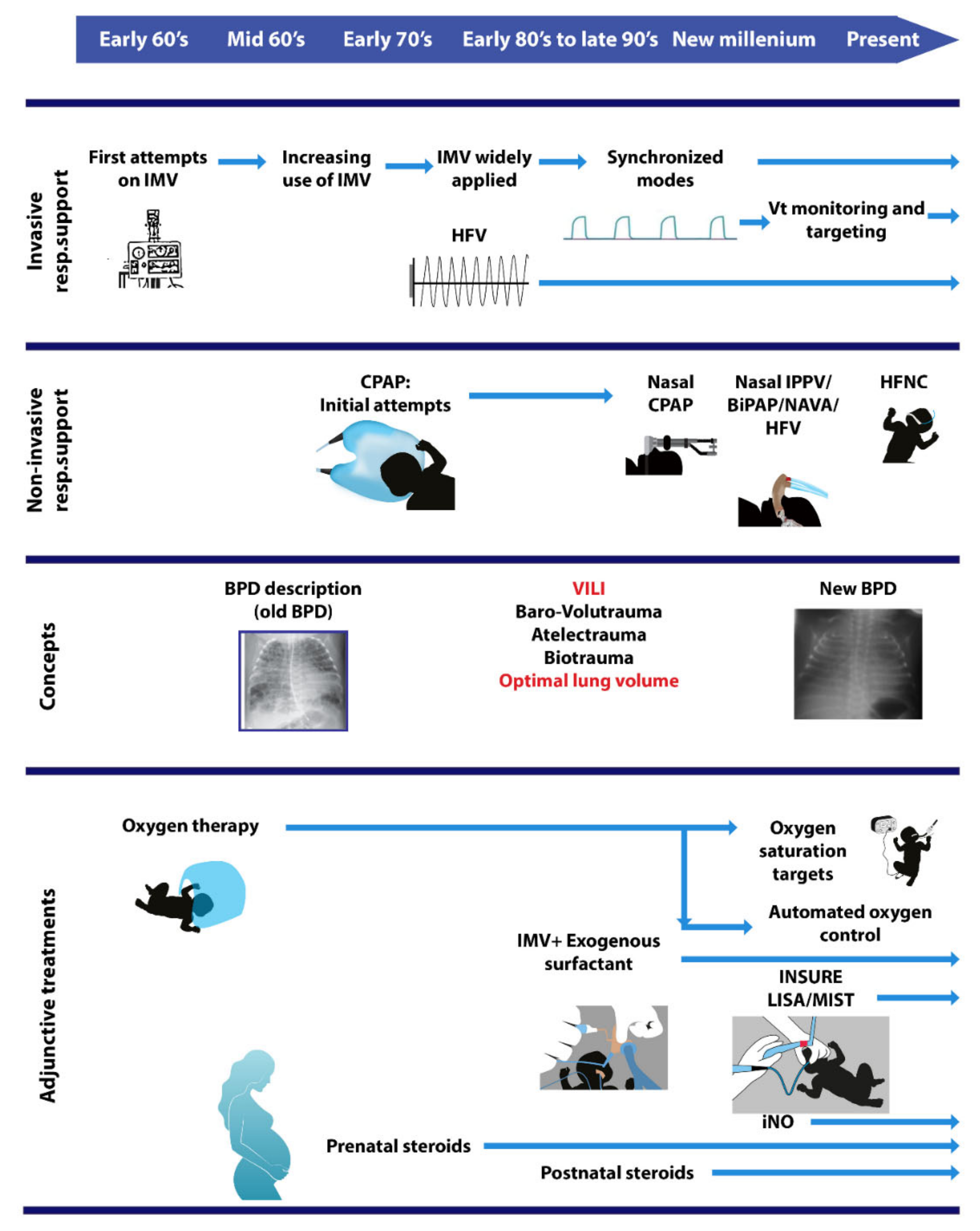
:1. Introduction
2. Effect of IMV on Neonatal Survival
3. Effect of Intertemporal Changes in IMV Associated Morbidities
3.1. Bronchopulmonary Dysplasia
3.2. Brain Injury
4. Most Important Developments in IMV and Their Effect on Outcomes
4.1. New Concepts
4.2. Modern Ventilators and New Modes of IMV
4.2.1. Synchronized Modes of IMV
4.2.2. Volume-Targeted Ventilation
4.2.3. High-Frequency Ventilation
5. Benefits from Moving toward Non-Invasive Respiratory Support
5.1. Non-Invasive Modes of Mechanical Ventilation
5.1.1. Nasal CPAP
5.1.2. Bi-Level Non-Invasive Ventilation
5.1.3. Nasal High-Frequency Ventilation
5.1.4. High-Flow Nasal Cannula
5.1.5. Nasal Neurally Adjusted Ventilatory Assist
5.2. Alternative Exogenous Surfactant Administration Techniques
5.2.1. Intubate-SURfactant-Extubate
5.2.2. Less Invasive Surfactant Administration/Minimal Invasive Surfactant Technique
5.2.3. Use of Nebulized Surfactant
6. The Role of the Adjunctive Pharmacological Interventions
6.1. Inhaled Nitric Oxide
6.2. Postnatal Steroids
6.3. Oxygen Therapy: Saturation Targets and Automated Control
7. Current Role of Invasive Mechanical Ventilation
8. Cost and Benefit of Neonatal Care-Respiratory Support
9. Future Challenges and Perspectives
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Henderson-Smart, D.J.; Wilkinson, A.R.; Raynes-Greenow, C.H. Mechanical Ventilation for Newborn Infants with Respiratory Failure Due to Pulmonary Disease. Cochrane Database Syst. Rev. 2002, 2002, CD002770. [Google Scholar] [CrossRef] [PubMed]
- Delivoria-Papadopoulos, M.; Swyer, P.R. Assisted Ventilation in Terminal Hyaline Membrane Disease. Arch. Dis. Child. 1964, 39, 481–484. [Google Scholar] [CrossRef] [Green Version]
- Philip, A.G.S. Historical Perspectives: Forty Years of Mechanical Ventilation …Then and Now…. NeoReviews 2003, 4, e335–e339. [Google Scholar] [CrossRef]
- Northway, W.H.; Rosan, R.C.; Porter, D.Y. Pulmonary Disease Following Respirator Therapy of Hyaline-Membrane Disease. Bronchopulmonary Dysplasia. N. Engl. J. Med. 1967, 276, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Gregory, G.A.; Kitterman, J.A.; Phibbs, R.H.; Tooley, W.H.; Hamilton, W.K. Treatment of the Idiopathic Respiratory-Distress Syndrome with Continuous Positive Airway Pressure. N. Engl. J. Med. 1971, 284, 1333–1340. [Google Scholar] [CrossRef]
- Liggins, G.C.; Howie, R.N. A Controlled Trial of Antepartum Glucocorticoid Treatment for Prevention of the Respiratory Distress Syndrome in Premature Infants. Pediatrics 1972, 50, 515–525. [Google Scholar]
- Fujiwara, T.; Maeta, H.; Chida, S.; Morita, T.; Watabe, Y.; Abe, T. Artificial Surfactant Therapy in Hyaline-Membrane Disease. Lancet 1980, 1, 55–59. [Google Scholar] [CrossRef]
- Hintz, S.R.; Poole, W.K.; Wright, L.L.; Fanaroff, A.A.; Kendrick, D.E.; Laptook, A.R.; Goldberg, R.; Duara, S.; Stoll, B.J.; Oh, W.; et al. Changes in Mortality and Morbidities among Infants Born at Less than 25 Weeks during the Post-Surfactant Era. Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, F128–F133. [Google Scholar] [CrossRef] [Green Version]
- Shah, P.S.; Sankaran, K.; Aziz, K.; Allen, A.C.; Seshia, M.; Ohlsson, A.; Lee, S.K. Canadian Neonatal Network Outcomes of Preterm Infants <29 Weeks Gestation over 10-Year Period in Canada: A Cause for Concern? J. Perinatol. Off. J. Calif. Perinat. Assoc. 2012, 32, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Kusuda, S.; Fujimura, M.; Uchiyama, A.; Totsu, S.; Matsunami, K. Neonatal Research Network, Japan Trends in Morbidity and Mortality among Very-Low-Birth-Weight Infants from 2003 to 2008 in Japan. Pediatr. Res. 2012, 72, 531–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horbar, J.D.; Carpenter, J.H.; Badger, G.J.; Kenny, M.J.; Soll, R.F.; Morrow, K.A.; Buzas, J.S. Mortality and Neonatal Morbidity among Infants 501 to 1500 Grams from 2000 to 2009. Pediatrics 2012, 129, 1019–1026. [Google Scholar] [CrossRef] [Green Version]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; Van Meurs, K.P.; Wyckoff, M.; et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015, 314, 1039–1051. [Google Scholar] [CrossRef] [Green Version]
- Donda, K.; Vijayakanthi, N.; Dapaah-Siakwan, F.; Bhatt, P.; Rastogi, D.; Rastogi, S. Trends in Epidemiology and Outcomes of Respiratory Distress Syndrome in the United States. Pediatr. Pulmonol. 2019, 54, 405–414. [Google Scholar] [CrossRef]
- Myrhaug, H.T.; Brurberg, K.G.; Hov, L.; Markestad, T. Survival and Impairment of Extremely Premature Infants: A Meta-Analysis. Pediatrics 2019, 143, e20180933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ancel, P.-Y.; Goffinet, F.; EPIPAGE-2 Writing Group; Kuhn, P.; Langer, B.; Matis, J.; Hernandorena, X.; Chabanier, P.; Joly-Pedespan, L.; Lecomte, B.; et al. Survival and Morbidity of Preterm Children Born at 22 through 34 Weeks’ Gestation in France in 2011: Results of the EPIPAGE-2 Cohort Study. JAMA Pediatr. 2015, 169, 230–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lui, K.; Lee, S.K.; Kusuda, S.; Adams, M.; Vento, M.; Reichman, B.; Darlow, B.A.; Lehtonen, L.; Modi, N.; Norman, M.; et al. Trends in Outcomes for Neonates Born Very Preterm and Very Low Birth Weight in 11 High-Income Countries. J. Pediatr. 2019, 215, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jobe, A.J. The New BPD: An Arrest of Lung Development. Pediatr. Res. 1999, 46, 641–643. [Google Scholar] [CrossRef] [Green Version]
- D’Apremont, I.; Marshall, G.; Musalem, C.; Mariani, G.; Musante, G.; Bancalari, A.; Fabres, J.; Mena, P.; Zegarra, J.; Tavosnanska, J.; et al. Trends in Perinatal Practices and Neonatal Outcomes of Very Low Birth Weight Infants during a 16-Year Period at NEOCOSUR Centers. J. Pediatr. 2020, 225, 44–50. [Google Scholar] [CrossRef]
- Siffel, C.; Kistler, K.D.; Lewis, J.F.M.; Sarda, S.P. Global Incidence of Bronchopulmonary Dysplasia among Extremely Preterm Infants: A Systematic Literature Review. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2021, 34, 1721–1731. [Google Scholar] [CrossRef] [Green Version]
- Avila-Alvarez, A.; Zozaya, C.; Pértega-Diaz, S.; Sanchez-Luna, M.; Iriondo-Sanz, M.; Elorza, M.D.; García-Muñoz Rodrigo, F. Spanish Neonatal Network SEN1500 Temporal Trends in Respiratory Care and Bronchopulmonary Dysplasia in Very Preterm Infants over a 10-Year Period in Spain. Available online: https://fn.bmj.com/content/early/2021/07/28/archdischild-2021-322402?utm_campaign=adcfn&utm_content=consumer&utm_medium=cpc&utm_source=trendmd&utm_term=usage-042019 (accessed on 23 August 2021).
- Barton, S.K.; Tolcos, M.; Miller, S.L.; Christoph-Roehr, C.; Schmölzer, G.M.; Moss, T.J.M.; Hooper, S.B.; Wallace, E.M.; Polglase, G.R. Ventilation-Induced Brain Injury in Preterm Neonates: A Review of Potential Therapies. Neonatology 2016, 110, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Vliegenthart, R.J.S.; van Kaam, A.H.; Aarnoudse-Moens, C.S.H.; van Wassenaer, A.G.; Onland, W. Duration of Mechanical Ventilation and Neurodevelopment in Preterm Infants. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F631–F635. [Google Scholar] [CrossRef]
- Choi, Y.-B.; Lee, J.; Park, J.; Jun, Y.H. Impact of Prolonged Mechanical Ventilation in Very Low Birth Weight Infants: Results From a National Cohort Study. J. Pediatr. 2018, 194, 34–39.e3. [Google Scholar] [CrossRef]
- Asztalos, E.V.; Church, P.T.; Riley, P.; Fajardo, C.; Shah, P.S. Canadian Neonatal Network and Canadian Neonatal Follow-up Network Investigators Neonatal Factors Associated with a Good Neurodevelopmental Outcome in Very Preterm Infants. Am. J. Perinatol. 2017, 34, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Greenough, A.; Rossor, T.E.; Sundaresan, A.; Murthy, V.; Milner, A.D. Synchronized Mechanical Ventilation for Respiratory Support in Newborn Infants. Cochrane Database Syst. Rev. 2016, 9, CD000456. [Google Scholar] [CrossRef]
- Stein, H.; Firestone, K. Application of Neurally Adjusted Ventilatory Assist in Neonates. Semin. Fetal. Neonatal Med. 2014, 19, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Rossor, T.E.; Hunt, K.A.; Shetty, S.; Greenough, A. Neurally Adjusted Ventilatory Assist Compared to Other Forms of Triggered Ventilation for Neonatal Respiratory Support. Cochrane Database Syst. Rev. 2017, 10, CD012251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallio, M.; Koskela, U.; Peltoniemi, O.; Kontiokari, T.; Pokka, T.; Suo-Palosaari, M.; Saarela, T. Neurally Adjusted Ventilatory Assist (NAVA) in Preterm Newborn Infants with Respiratory Distress Syndrome-a Randomized Controlled Trial. Eur. J. Pediatr. 2016, 175, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.A.; Dassios, T.; Greenough, A. Proportional Assist Ventilation (PAV) versus Neurally Adjusted Ventilator Assist (NAVA): Effect on Oxygenation in Infants with Evolving or Established Bronchopulmonary Dysplasia. Eur. J. Pediatr. 2020, 179, 901–908. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, K.I.; Klingenberg, C.; Morley, C.J.; Davis, P.G. Volume-Targeted versus Pressure-Limited Ventilation for Preterm Infants: A Systematic Review and Meta-Analysis. Neonatology 2011, 100, 219–227. [Google Scholar] [CrossRef]
- Klingenberg, C.; Wheeler, K.I.; McCallion, N.; Morley, C.J.; Davis, P.G. Volume-Targeted versus Pressure-Limited Ventilation in Neonates. Cochrane Database Syst. Rev. 2017, 10, CD003666. [Google Scholar] [CrossRef]
- Wang, C.; Guo, L.; Chi, C.; Wang, X.; Guo, L.; Wang, W.; Zhao, N.; Wang, Y.; Zhang, Z.; Li, E. Mechanical Ventilation Modes for Respiratory Distress Syndrome in Infants: A Systematic Review and Network Meta-Analysis. Crit. Care 2015, 19, 108. [Google Scholar] [CrossRef] [Green Version]
- Cools, F.; Offringa, M.; Askie, L.M. Elective High Frequency Oscillatory Ventilation versus Conventional Ventilation for Acute Pulmonary Dysfunction in Preterm Infants. Cochrane Database Syst. Rev. 2015, 3, CD000104. [Google Scholar] [CrossRef]
- Zivanovic, S.; Peacock, J.; Alcazar-Paris, M.; Lo, J.W.; Lunt, A.; Marlow, N.; Calvert, S.; Greenough, A. Late Outcomes of a Randomized Trial of High-Frequency Oscillation in Neonates. N. Engl. J. Med. 2014, 370, 1121–1130. [Google Scholar] [CrossRef] [Green Version]
- Carney, D.; DiRocco, J.; Nieman, G. Dynamic Alveolar Mechanics and Ventilator-Induced Lung Injury. Crit. Care Med. 2005, 33, S122–S128. [Google Scholar] [CrossRef]
- Askie, L.M.; Darlow, B.A.; Davis, P.G.; Finer, N.; Stenson, B.; Vento, M.; Whyte, R. Effects of Targeting Lower versus Higher Arterial Oxygen Saturations on Death or Disability in Preterm Infants. Cochrane Database Syst. Rev. 2017, 4, CD011190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturrock, S.; Ambulkar, H.; Williams, E.E.; Sweeney, S.; Bednarczuk, N.F.; Dassios, T.; Greenough, A. A Randomised Crossover Trial of Closed Loop Automated Oxygen Control in Preterm, Ventilated Infants. Acta Paediatr. 2021, 110, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Schmölzer, G.M.; Kumar, M.; Pichler, G.; Aziz, K.; O’Reilly, M.; Cheung, P.-Y. Non-Invasive versus Invasive Respiratory Support in Preterm Infants at Birth: Systematic Review and Meta-Analysis. BMJ 2013, 347, f5980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, H.S.; Bührer, C. Avoiding Endotracheal Ventilation to Prevent Bronchopulmonary Dysplasia: A Meta-Analysis. Pediatrics 2013, 132, e1351–e1360. [Google Scholar] [CrossRef] [Green Version]
- Lemyre, B.; Laughon, M.; Bose, C.; Davis, P.G. Early Nasal Intermittent Positive Pressure Ventilation (NIPPV) versus Early Nasal Continuous Positive Airway Pressure (NCPAP) for Preterm Infants. Cochrane Database Syst. Rev. 2016, 12, CD005384. [Google Scholar] [CrossRef] [PubMed]
- Lemyre, B.; Davis, P.G.; De Paoli, A.G.; Kirpalani, H. Nasal Intermittent Positive Pressure Ventilation (NIPPV) versus Nasal Continuous Positive Airway Pressure (NCPAP) for Preterm Neonates after Extubation. Cochrane Database Syst. Rev. 2017, 2, CD003212. [Google Scholar] [CrossRef]
- Solevåg, A.L.; Cheung, P.-Y.; Schmölzer, G.M. Bi-Level Noninvasive Ventilation in Neonatal Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Neonatology 2021, 118, 1–10. [Google Scholar] [CrossRef]
- Iranpour, R.; Armanian, A.-M.; Abedi, A.-R.; Farajzadegan, Z. Nasal High-Frequency Oscillatory Ventilation (NHFOV) versus Nasal Continuous Positive Airway Pressure (NCPAP) as an Initial Therapy for Respiratory Distress Syndrome (RDS) in Preterm and near-Term Infants. BMJ Paediatr. Open 2019, 3, e000443. [Google Scholar] [CrossRef] [Green Version]
- Malakian, A.; Bashirnezhadkhabaz, S.; Aramesh, M.-R.; Dehdashtian, M. Noninvasive High-Frequency Oscillatory Ventilation versus Nasal Continuous Positive Airway Pressure in Preterm Infants with Respiratory Distress Syndrome: A Randomized Controlled Trial. J. Matern. Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2020, 33, 2601–2607. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Huang, X.; Zhang, Z. Noninvasive High-Frequency Oscillatory Ventilation as Respiratory Support in Preterm Infants: A Meta-Analysis of Randomized Controlled Trials. Respir. Res. 2019, 20, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, D.; Andersen, C.; O’Donnell, C.P.F.; De Paoli, A.G.; Manley, B.J. High Flow Nasal Cannula for Respiratory Support in Preterm Infants. Cochrane Database Syst. Rev. 2016, 2, CD006405. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.T.; Owen, L.S.; Manley, B.J.; Frøisland, D.H.; Donath, S.M.; Dalziel, K.M.; Pritchard, M.A.; Cartwright, D.W.; Collins, C.L.; Malhotra, A.; et al. Nasal High-Flow Therapy for Primary Respiratory Support in Preterm Infants. N. Engl. J. Med. 2016, 375, 1142–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, H.; Li, X.-X.; Li, J.; Zhang, Z.-Q. High-Flow Nasal Cannula versus Nasal Continuous Positive Airway Pressure for Respiratory Support in Preterm Infants: A Meta-Analysis of Randomized Controlled Trials. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2021, 34, 259–266. [Google Scholar] [CrossRef]
- Yagui, A.C.; Meneses, J.; Zólio, B.A.; Brito, G.M.G.; da Silva, R.J.; Rebello, C.M. Nasal Continuous Positive Airway Pressure (NCPAP) or Noninvasive Neurally Adjusted Ventilatory Assist (NIV-NAVA) for Preterm Infants with Respiratory Distress after Birth: A Randomized Controlled Trial. Pediatr. Pulmonol. 2019, 54, 1704–1711. [Google Scholar] [CrossRef]
- Kallio, M.; Mahlman, M.; Koskela, U.; Aikio, O.; Suo-Palosaari, M.; Pokka, T.; Saarela, T.; Hallman, M. NIV NAVA versus Nasal CPAP in Premature Infants: A Randomized Clinical Trial. Neonatology 2019, 116, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Mari, J.; Franczia, P.; Margas, W.; Rutkowski, J.; Bebrysz, M.; Bokiniec, R.; Seliga-Siwecka, J. International Consensus Is needed on Premedication for Non-Emergency Neonatal Intubation after Survey Found Wide-Ranging Policies and Practices in 70 Countries. Acta Paediatr. 2020, 109, 1369–1375. [Google Scholar] [CrossRef]
- De Bisschop, B.; Derriks, F.; Cools, F. Early Predictors for INtubation-SURfactant-Extubation Failure in Preterm Infants with Neonatal Respiratory Distress Syndrome: A Systematic Review. Neonatology 2020, 117, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Aldana-Aguirre, J.C.; Pinto, M.; Featherstone, R.M.; Kumar, M. Less Invasive Surfactant Administration versus Intubation for Surfactant Delivery in Preterm Infants with Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F17–F23. [Google Scholar] [CrossRef]
- Brasher, M.; Raffay, T.M.; Cunningham, M.D.; Abu Jawdeh, E.G. Aerosolized Surfactant for Preterm Infants with Respiratory Distress Syndrome. Children 2021, 8, 493. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.J.; Gerday, E.; Minton, S.; Katheria, A.; Albert, G.; Flores-Torres, J.; Famuyide, M.; Lampland, A.; Guthrie, S.; Kuehn, D.; et al. Aerosolized Calfactant for Newborns With Respiratory Distress: A Randomized Trial. Pediatrics 2020, 146, e20193967. [Google Scholar] [CrossRef]
- Gaertner, V.D.; Bassler, D.; Rüegger, C.M. Does Surfactant Nebulization Prevent Early Intubation in Preterm Infants? A Protocol for a Systematic Review and Meta-Analysis. Syst. Rev. 2021, 10, 9. [Google Scholar] [CrossRef]
- Barrington, K.J.; Finer, N.; Pennaforte, T. Inhaled Nitric Oxide for Respiratory Failure in Preterm Infants. Cochrane Database Syst. Rev. 2017, 1, CD000509. [Google Scholar] [CrossRef] [PubMed]
- Mourani, P.M.; Abman, S.H. Pulmonary Hypertension and Vascular Abnormalities in Bronchopulmonary Dysplasia. Clin. Perinatol. 2015, 42, 839–855. [Google Scholar] [CrossRef] [Green Version]
- Doyle, L.W.; Cheong, J.L.; Ehrenkranz, R.A.; Halliday, H.L. Late (>7 Days) Systemic Postnatal Corticosteroids for Prevention of Bronchopulmonary Dysplasia in Preterm Infants. Cochrane Database Syst. Rev. 2017, 10, CD001145. [Google Scholar] [CrossRef] [PubMed]
- Baud, O.; Maury, L.; Lebail, F.; Ramful, D.; El Moussawi, F.; Nicaise, C.; Zupan-Simunek, V.; Coursol, A.; Beuchée, A.; Bolot, P.; et al. Effect of Early Low-Dose Hydrocortisone on Survival without Bronchopulmonary Dysplasia in Extremely Preterm Infants (PREMILOC): A Double-Blind, Placebo-Controlled, Multicentre, Randomised Trial. Lancet 2016, 387, 1827–1836. [Google Scholar] [CrossRef]
- Shah, S.S.; Ohlsson, A.; Halliday, H.L.; Shah, V.S. Inhaled versus Systemic Corticosteroids for Preventing Bronchopulmonary Dysplasia in Ventilated Very Low Birth Weight Preterm Neonates. Cochrane Database Syst. Rev. 2017, 10, CD002058. [Google Scholar] [CrossRef]
- ANZCTR: ACTRN12617000322336. Available online: https://www.anzctr.org.au (accessed on 10 September 2021).
- BOOST II United Kingdom Collaborative Group; BOOST II Australia Collaborative Group; BOOST II New Zealand Collaborative Group; Stenson, B.J.; Tarnow-Mordi, W.O.; Darlow, B.A.; Simes, J.; Juszczak, E.; Askie, L.; Battin, M.; et al. Oxygen Saturation and Outcomes in Preterm Infants. N. Engl. J. Med. 2013, 368, 2094–2104. [Google Scholar] [CrossRef] [Green Version]
- Salverda, H.H.; Oldenburger, N.J.; Rijken, M.; Pauws, S.C.; Dargaville, P.A.; Te Pas, A.B. The Effect of Automated Oxygen Control on Clinical Outcomes in Preterm Infants: A Pre- and Post-Implementation Cohort Study. Eur. J. Pediatr. 2021. [Google Scholar] [CrossRef]
- Srivatsa, B.; Malcolm, K.; Clark, R.H.; Kupke, K.G. Effect of a Novel Oxygen Saturation Targeting Strategy on Mortality, Retinopathy of Prematurity, and Bronchopulmonary Dysplasia in Neonates Born Extremely Preterm. J. Pediatr. 2021, 234, 33–37. [Google Scholar] [CrossRef]
- Dreyfuss, D.; Soler, P.; Basset, G.; Saumon, G. High Inflation Pressure Pulmonary Edema. Respective Effects of High Airway Pressure, High Tidal Volume, and Positive End-Expiratory Pressure. Am. Rev. Respir. Dis. 1988, 137, 1159–1164. [Google Scholar] [CrossRef]
- Amato, M.B.; Barbas, C.S.; Medeiros, D.M.; Magaldi, R.B.; Schettino, G.P.; Lorenzi-Filho, G.; Kairalla, R.A.; Deheinzelin, D.; Munoz, C.; Oliveira, R.; et al. Effect of a Protective-Ventilation Strategy on Mortality in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 1998, 338, 347–354. [Google Scholar] [CrossRef]
- Van Kaam, A.H.; De Luca, D.; Hentschel, R.; Hutten, J.; Sindelar, R.; Thome, U.; Zimmermann, L.J.I. Modes and Strategies for Providing Conventional Mechanical Ventilation in Neonates. Pediatr. Res. 2019, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Van Kaam, A.H.; Rimensberger, P.C.; Borensztajn, D.; De Jaegere, A.P. Neovent Study Group Ventilation Practices in the Neonatal Intensive Care Unit: A Cross-Sectional Study. J. Pediatr. 2010, 157, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Garland, J.S.; Buck, R.K.; Allred, E.N.; Leviton, A. Hypocarbia before Surfactant Therapy Appears to Increase Bronchopulmonary Dysplasia Risk in Infants with Respiratory Distress Syndrome. Arch. Pediatr. Adolesc. Med. 1995, 149, 617–622. [Google Scholar] [CrossRef]
- Graziani, L.J.; Spitzer, A.R.; Mitchell, D.G.; Merton, D.A.; Stanley, C.; Robinson, N.; McKee, L. Mechanical Ventilation in Preterm Infants: Neurosonographic and Developmental Studies. Pediatrics 1992, 90, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Woodgate, P.G.; Davies, M.W. Permissive Hypercapnia for the Prevention of Morbidity and Mortality in Mechanically Ventilated Newborn Infants. Available online: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002061/abstract#CD002061-abs-0001 (accessed on 12 August 2021).
- Thome, U.H.; Genzel-Boroviczeny, O.; Bohnhorst, B.; Schmid, M.; Fuchs, H.; Rohde, O.; Avenarius, S.; Topf, H.-G.; Zimmermann, A.; Faas, D.; et al. Permissive Hypercapnia in Extremely Low Birthweight Infants (PHELBI): A Randomised Controlled Multicentre Trial. Lancet Respir. Med. 2015, 3, 534–543. [Google Scholar] [CrossRef]
- Thome, U.H.; Genzel-Boroviczeny, O.; Bohnhorst, B.; Schmid, M.; Fuchs, H.; Rohde, O.; Avenarius, S.; Topf, H.-G.; Zimmermann, A.; Faas, D.; et al. Neurodevelopmental Outcomes of Extremely Low Birthweight Infants Randomised to Different PCO2 Targets: The PHELBI Follow-up Study. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F376–F382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, S.K.; Lee, J.; Jun, Y.H. Neural Feedback Is Insufficient in Preterm Infants during Neurally Adjusted Ventilatory Assist. Pediatr. Pulmonol. 2019, 54, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.K.; Donn, S.M.; Gavey, J.; McCarty, M. Randomised Trial of Volume Controlled versus Time Cycled, Pressure Limited Ventilation in Preterm Infants with Respiratory Distress Syndrome. Arch. Dis. Child. Fetal Neonatal Ed. 1997, 77, F202–F205. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Keszler, M. Survey of Ventilation Practices in the Neonatal Intensive Care Units of the United States and Canada: Use of Volume-Targeted Ventilation and Barriers to Its Use. Am. J. Perinatol. 2019, 36, 484–489. [Google Scholar] [CrossRef]
- Keszler, M. Volume-Targeted Ventilation: One Size Does Not Fit All. Evidence-Based Recommendations for Successful Use. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F108–F112. [Google Scholar] [CrossRef]
- Klingenberg, C.; Wheeler, K.I.; Davis, P.G.; Morley, C.J. A Practical Guide to Neonatal Volume Guarantee Ventilation. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2011, 31, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Keszler, M.; Nassabeh-Montazami, S.; Abubakar, K. Evolution of Tidal Volume Requirement during the First 3 Weeks of Life in Infants <800 g Ventilated with Volume Guarantee. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F279–F282. [Google Scholar] [CrossRef]
- Hunt, K.; Dassios, T.; Ali, K.; Greenough, A. Volume Targeting Levels and Work of Breathing in Infants with Evolving or Established Bronchopulmonary Dysplasia. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F46–F49. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.; Wang, H.; Tepper, R.; Sokol, G.M.; Rose, R. Expired Tidal Volume Variation in Extremely Low Birth Weight and Very Low Birth Weight Infants on Volume-Targeted Ventilation. J. Pediatr. 2019, 207, 248–251. [Google Scholar] [CrossRef]
- Wallström, L.; Sjöberg, A.; Sindelar, R. Early Volume Targeted Ventilation in Preterm Infants Born at 22–25 Weeks of Gestational Age. Pediatr. Pulmonol. 2021, 56, 1000–1007. [Google Scholar] [CrossRef]
- Keszler, M.; Durand, D.J. Neonatal High-Frequency Ventilation. Past, Present, and Future. Clin. Perinatol. 2001, 28, 579–607. [Google Scholar] [CrossRef]
- Clark, R.H.; Gerstmann, D.R.; Null, D.M.; deLemos, R.A. Prospective Randomized Comparison of High-Frequency Oscillatory and Conventional Ventilation in Respiratory Distress Syndrome. Pediatrics 1992, 89, 5–12. [Google Scholar] [PubMed]
- Lachmann, B. Open up the Lung and Keep the Lung Open. Intensive Care Med. 1992, 18, 319–321. [Google Scholar] [CrossRef] [Green Version]
- Thome, U.H.; Carlo, W.A.; Pohlandt, F. Ventilation Strategies and Outcome in Randomised Trials of High Frequency Ventilation. Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, F466–F473. [Google Scholar] [CrossRef] [Green Version]
- Hsu, J.-F.; Yang, M.-C.; Chu, S.-M.; Yang, L.-Y.; Chiang, M.-C.; Lai, M.-Y.; Huang, H.-R.; Pan, Y.-B.; Fu, R.-H.; Tsai, M.-H. Therapeutic Effects and Outcomes of Rescue High-Frequency Oscillatory Ventilation for Premature Infants with Severe Refractory Respiratory Failure. Sci. Rep. 2021, 11, 8471. [Google Scholar] [CrossRef]
- Bhuta, T.; Henderson-Smart, D.J. Rescue High Frequency Oscillatory Ventilation versus Conventional Ventilation for Pulmonary Dysfunction in Preterm Infants. Cochrane Database Syst. Rev. 2000, 2, CD000438. [Google Scholar] [CrossRef]
- Sánchez-Luna, M.; González-Pacheco, N.; Belik, J.; Santos, M.; Tendillo, F. New Ventilator Strategies: High-Frequency Oscillatory Ventilation Combined with Volume Guarantee. Am. J. Perinatol. 2018, 35, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Tuzun, F.; Deliloglu, B.; Cengiz, M.M.; Iscan, B.; Duman, N.; Ozkan, H. Volume Guarantee High-Frequency Oscillatory Ventilation in Preterm Infants With RDS: Tidal Volume and DCO2 Levels for Optimal Ventilation Using Open-Lung Strategies. Front. Pediatr. 2020, 8, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iscan, B.; Duman, N.; Tuzun, F.; Kumral, A.; Ozkan, H. Impact of Volume Guarantee on High-Frequency Oscillatory Ventilation in Preterm Infants: A Randomized Crossover Clinical Trial. Neonatology 2015, 108, 277–282. [Google Scholar] [CrossRef]
- Belteki, G.; Morley, C.J. High-Frequency Oscillatory Ventilation with Volume Guarantee: A Single-Centre Experience. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F384–F389. [Google Scholar] [CrossRef]
- Chavez, T.A.; Lakshmanan, A.; Figueroa, L.; Iyer, N.; Stavroudis, T.A.; Garingo, A.; Friedlich, P.S.; Ramanathan, R. Resource Utilization Patterns Using Non-Invasive Ventilation in Neonates with Respiratory Distress Syndrome. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2018, 38, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Morley, C.J.; Davis, P.G.; Doyle, L.W.; Brion, L.P.; Hascoet, J.-M.; Carlin, J.B. COIN Trial Investigators Nasal CPAP or Intubation at Birth for Very Preterm Infants. N. Engl. J. Med. 2008, 358, 700–708. [Google Scholar] [CrossRef] [Green Version]
- SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network; Finer, N.N.; Carlo, W.A.; Walsh, M.C.; Rich, W.; Gantz, M.G.; Laptook, A.R.; Yoder, B.A.; Faix, R.G.; Das, A.; et al. Early CPAP versus Surfactant in Extremely Preterm Infants. N. Engl. J. Med. 2010, 362, 1970–1979. [Google Scholar] [CrossRef] [Green Version]
- Dunn, M.S.; Kaempf, J.; de Klerk, A.; de Klerk, R.; Reilly, M.; Howard, D.; Ferrelli, K.; O’Conor, J.; Soll, R.F. Vermont Oxford Network DRM Study Group Randomized Trial Comparing 3 Approaches to the Initial Respiratory Management of Preterm Neonates. Pediatrics 2011, 128, e1069–e1076. [Google Scholar] [CrossRef]
- Avery, M.E.; Tooley, W.H.; Keller, J.B.; Hurd, S.S.; Bryan, M.H.; Cotton, R.B.; Epstein, M.F.; Fitzhardinge, P.M.; Hansen, C.B.; Hansen, T.N. Is Chronic Lung Disease in Low Birth Weight Infants Preventable? A Survey of Eight Centers. Pediatrics 1987, 79, 26–30. [Google Scholar] [PubMed]
- Alexiou, S.; Panitch, H.B. Physiology of Non-Invasive Respiratory Support. Semin. Fetal. Neonatal Med. 2016, 21, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, P.; Ho, J.J.; Davis, P.G. Prophylactic Nasal Continuous Positive Airway Pressure for Preventing Morbidity and Mortality in Very Preterm Infants. Cochrane Database Syst. Rev. 2016, 6, CD001243. [Google Scholar] [CrossRef]
- Shaffer, T.H.; Alapati, D.; Greenspan, J.S.; Wolfson, M.R. Neonatal Non-Invasive Respiratory Support: Physiological Implications. Pediatr. Pulmonol. 2012, 47, 837–847. [Google Scholar] [CrossRef] [Green Version]
- Dumpa, V.; Bhandari, V. Non-Invasive Ventilatory Strategies to Decrease Bronchopulmonary Dysplasia-Where Are We in 2021? Children 2021, 8, 132. [Google Scholar] [CrossRef]
- Migliori, C.; Motta, M.; Angeli, A.; Chirico, G. Nasal Bilevel vs. Continuous Positive Airway Pressure in Preterm Infants. Pediatr. Pulmonol. 2005, 40, 426–430. [Google Scholar] [CrossRef]
- Ricotti, A.; Salvo, V.; Zimmermann, L.J.I.; Gavilanes, A.W.D.; Barberi, I.; Lista, G.; Colivicchi, M.; Temporini, F.; Gazzolo, D. N-SIPPV versus Bi-Level N-CPAP for Early Treatment of Respiratory Distress Syndrome in Preterm Infants. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2013, 26, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Rong, Z.-H.; Li, W.-B.; Liu, W.; Cai, B.-H.; Wang, J.; Yang, M.; Li, W.; Chang, L.-W. Nasal Bi-Level Positive Airway Pressure (BiPAP) versus Nasal Continuous Positive Airway Pressure (CPAP) in Preterm Infants ≤32 Weeks: A Retrospective Cohort Study. J. Paediatr. Child. Health 2016, 52, 493–498. [Google Scholar] [CrossRef]
- Lee, M.-J.; Choi, E.K.; Park, K.H.; Shin, J.; Choi, B.M. Effectiveness of NCPAP for Moderate Preterm Infants Compared to BiPAP: A Randomized, Controlled Non-Inferiority Trial. Pediatr. Int. Off. J. Jpn. Pediatr. Soc. 2020, 62, 59–64. [Google Scholar] [CrossRef]
- Capasso, L.; Borrelli, A.C.; Cerullo, J.; Caiazzo, M.A.; Coppola, C.; Palma, M.; Raimondi, F. Reducing Post-Extubation Failure Rates in Very Preterm Infants: Is BiPAP Better than CPAP? J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- De Luca, D.; Dell’Orto, V. Non-Invasive High-Frequency Oscillatory Ventilation in Neonates: Review of Physiology, Biology and Clinical Data. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F565–F570. [Google Scholar] [CrossRef] [Green Version]
- Mukerji, A.; Dunn, M. High-Frequency Ventilation as a Mode of Noninvasive Respiratory Support. Clin. Perinatol. 2016, 43, 725–740. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Ma, J.; Feng, Z.; Li, J.; Shi, Y. Nasal High-Frequency Oscillatory Ventilation in Preterm Infants With Respiratory Distress Syndrome and ARDS After Extubation: A Randomized Controlled Trial. Chest 2019, 155, 740–748. [Google Scholar] [CrossRef]
- Rüegger, C.M.; Lorenz, L.; Kamlin, C.O.F.; Manley, B.J.; Owen, L.S.; Bassler, D.; Tingay, D.G.; Donath, S.M.; Davis, P.G. The Effect of Noninvasive High-Frequency Oscillatory Ventilation on Desaturations and Bradycardia in Very Preterm Infants: A Randomized Crossover Trial. J. Pediatr. 2018, 201, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, V.D.; Waldmann, A.D.; Davis, P.G.; Bassler, D.; Springer, L.; Thomson, J.; Tingay, D.G.; Rüegger, C.M. Transmission of Oscillatory Volumes into the Preterm Lung during Noninvasive High-Frequency Ventilation. Am. J. Respir. Crit. Care Med. 2021, 203, 998–1005. [Google Scholar] [CrossRef]
- Shi, Y.; De Luca, D. NASal OscillatioN post-Extubation (NASONE) study group Continuous Positive Airway Pressure (CPAP) vs Noninvasive Positive Pressure Ventilation (NIPPV) vs Noninvasive High Frequency Oscillation Ventilation (NHFOV) as Post-Extubation Support in Preterm Neonates: Protocol for an Assessor-Blinded, Multicenter, Randomized Controlled Trial. BMC Pediatr. 2019, 19, 256. [Google Scholar] [CrossRef] [Green Version]
- Manley, B.J.; Owen, L.S. High-Flow Nasal Cannula: Mechanisms, Evidence and Recommendations. Semin. Fetal. Neonatal Med. 2016, 21, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Murki, S.; Singh, J.; Khant, C.; Kumar Dash, S.; Oleti, T.P.; Joy, P.; Kabra, N.S. High-Flow Nasal Cannula versus Nasal Continuous Positive Airway Pressure for Primary Respiratory Support in Preterm Infants with Respiratory Distress: A Randomized Controlled Trial. Neonatology 2018, 113, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Muhsen, W.; Roy, R. A Comparative Study of HHHFNC and NCPAP in Preventing Reintubation in Extreme Preterm Infants Born at Less than 30-Week Gestation. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2018, 31, 3197–3200. [Google Scholar] [CrossRef]
- Van Delft, B.; Van Ginderdeuren, F.; Lefevere, J.; van Delft, C.; Cools, F. Weaning Strategies for the Withdrawal of Non-Invasive Respiratory Support Applying Continuous Positive Airway Pressure in Preterm Infants: A Systematic Review and Meta-Analysis. BMJ Paediatr. Open 2020, 4, e000858. [Google Scholar] [CrossRef] [PubMed]
- Verder, H.; Agertoft, L.; Albertsen, P.; Christensen, N.C.; Curstedt, T.; Ebbesen, F.; Greisen, G.; Hobolth, N.; Holm, V.; Jacobsen, T. Surfactant treatment of newborn infants with respiratory distress syndrome primarily treated with nasal continuous positive air pressure. A pilot study. Ugeskr. Laeger 1992, 154, 2136–2139. [Google Scholar] [PubMed]
- Kribs, A.; Pillekamp, F.; Hünseler, C.; Vierzig, A.; Roth, B. Early Administration of Surfactant in Spontaneous Breathing with NCPAP: Feasibility and Outcome in Extremely Premature Infants (Postmenstrual Age </=27 Weeks). Paediatr. Anaesth. 2007, 17, 364–369. [Google Scholar] [CrossRef]
- O’Donnell, C.P.F.; Kamlin, C.O.F.; Davis, P.G.; Morley, C.J. Endotracheal Intubation Attempts during Neonatal Resuscitation: Success Rates, Duration, and Adverse Effects. Pediatrics 2006, 117, e16–e21. [Google Scholar] [CrossRef] [Green Version]
- Dargaville, P.A.; Kamlin, C.O.F.; De Paoli, A.G.; Carlin, J.B.; Orsini, F.; Soll, R.F.; Davis, P.G. The OPTIMIST-A Trial: Evaluation of Minimally-Invasive Surfactant Therapy in Preterm Infants 25–28 Weeks Gestation. BMC Pediatr. 2014, 14, 213. [Google Scholar] [CrossRef] [Green Version]
- Herting, E.; Härtel, C.; Göpel, W. Less Invasive Surfactant Administration: Best Practices and Unanswered Questions. Curr. Opin. Pediatr. 2020, 32, 228–234. [Google Scholar] [CrossRef]
- Conlon, S.M.; Osborne, A.; Bodie, J.; Marasch, J.; Ryan, R.M.; Glenn, T. Introducing Less-Invasive Surfactant Administration into a Level IV NICU: A Quality Improvement Initiative. Children 2021, 8, 580. [Google Scholar] [CrossRef]
- Isayama, T.; Iwami, H.; McDonald, S.; Beyene, J. Association of Noninvasive Ventilation Strategies With Mortality and Bronchopulmonary Dysplasia Among Preterm Infants: A Systematic Review and Meta-Analysis. JAMA 2016, 316, 611–624. [Google Scholar] [CrossRef] [Green Version]
- Mehler, K.; Broer, A.; Roll, C.; Göpel, W.; Wieg, C.; Jahn, P.; Teig, N.; Höhn, T.; Welzing, L.; Vochem, M.; et al. Developmental Outcome of Extremely Preterm Infants Is Improved after Less Invasive Surfactant Application: Developmental Outcome after LISA. Acta Paediatr. 2021, 110, 818–825. [Google Scholar] [CrossRef]
- Abdel-Latif, M.E.; Davis, P.G.; Wheeler, K.I.; De Paoli, A.G.; Dargaville, P.A. Surfactant Therapy via Thin Catheter in Preterm Infants with or at Risk of Respiratory Distress Syndrome. Cochrane Database Syst. Rev. 2021, 5, CD011672. [Google Scholar] [CrossRef]
- Roberts, K.; Stepanovich, G.; Bhatt-Mehta, V.; Donn, S.M. New Pharmacologic Approaches to Bronchopulmonary Dysplasia. J. Exp. Pharmacol. 2021, 13, 377–396. [Google Scholar] [CrossRef]
- Barrington, K.J.; Finer, N.; Pennaforte, T.; Altit, G. Nitric Oxide for Respiratory Failure in Infants Born at or near Term. Cochrane Database Syst. Rev. 2017, 1, CD000399. [Google Scholar] [CrossRef]
- Mammel, M.C.; Green, T.P.; Johnson, D.E.; Thompson, T.R. Controlled Trial of Dexamethasone Therapy in Infants with Bronchopulmonary Dysplasia. Lancet 1983, 1, 1356–1358. [Google Scholar] [CrossRef]
- Doyle, L.W.; Cheong, J.L.; Ehrenkranz, R.A.; Halliday, H.L. Early (<8 Days) Systemic Postnatal Corticosteroids for Prevention of Bronchopulmonary Dysplasia in Preterm Infants. Cochrane Database Syst. Rev. 2017, 10, CD001146. [Google Scholar] [CrossRef]
- Onland, W.; Cools, F.; Kroon, A.; Rademaker, K.; Merkus, M.P.; Dijk, P.H.; van Straaten, H.L.; Te Pas, A.B.; Mohns, T.; Bruneel, E.; et al. Effect of Hydrocortisone Therapy Initiated 7 to 14 Days After Birth on Mortality or Bronchopulmonary Dysplasia Among Very Preterm Infants Receiving Mechanical Ventilation: A Randomized Clinical Trial. JAMA 2019, 321, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Bassler, D.; Plavka, R.; Shinwell, E.S.; Hallman, M.; Jarreau, P.-H.; Carnielli, V.; Van den Anker, J.N.; Meisner, C.; Engel, C.; Schwab, M.; et al. Early Inhaled Budesonide for the Prevention of Bronchopulmonary Dysplasia. N. Engl. J. Med. 2015, 373, 1497–1506. [Google Scholar] [CrossRef] [Green Version]
- Mitra, S.; McMillan, D. Automated Control of Fraction of Inspired Oxygen: Is It Time for Widespread Adoption? Curr. Opin. Pediatr. 2021, 33, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Maiwald, C.A.; Niemarkt, H.J.; Poets, C.F.; Urschitz, M.S.; König, J.; Hummler, H.; Bassler, D.; Engel, C.; Franz, A.R. FiO2-C Study Group Effects of Closed-Loop Automatic Control of the Inspiratory Fraction of Oxygen (FiO2-C) on Outcome of Extremely Preterm Infants—Study Protocol of a Randomized Controlled Parallel Group Multicenter Trial for Safety and Efficacy. BMC Pediatr. 2019, 19, 363. [Google Scholar] [CrossRef] [Green Version]
- Dargaville, P.A.; Gerber, A.; Johansson, S.; De Paoli, A.G.; Kamlin, C.O.F.; Orsini, F.; Davis, P.G. Australian and New Zealand Neonatal Network Incidence and Outcome of CPAP Failure in Preterm Infants. Pediatrics 2016, 138, e20153985. [Google Scholar] [CrossRef] [Green Version]
- Dargaville, P.A.; Aiyappan, A.; De Paoli, A.G.; Dalton, R.G.B.; Kuschel, C.A.; Kamlin, C.O.; Orsini, F.; Carlin, J.B.; Davis, P.G. Continuous Positive Airway Pressure Failure in Preterm Infants: Incidence, Predictors and Consequences. Neonatology 2013, 104, 8–14. [Google Scholar] [CrossRef]
- De Luca, D.; Autilio, C.; Pezza, L.; Shankar-Aguilera, S.; Tingay, D.G.; Carnielli, V.P. Personalized Medicine for the Management of RDS in Preterm Neonates. Neonatology 2021, 1–12. [Google Scholar] [CrossRef]
- Patel, R.M.; Kandefer, S.; Walsh, M.C.; Bell, E.F.; Carlo, W.A.; Laptook, A.R.; Sánchez, P.J.; Shankaran, S.; Van Meurs, K.P.; Ball, M.B.; et al. Causes and Timing of Death in Extremely Premature Infants from 2000 through 2011. N. Engl. J. Med. 2015, 372, 331–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm Birth: Causes, Consequences, and Prevention; Behrman, R.E., Butler, A.S., Eds.; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press: Washington, DC, USA, 2007; ISBN 978-0-309-10159-2. [Google Scholar]
- Waitzman, N.J.; Jalali, A.; Grosse, S.D. Preterm Birth Lifetime Costs in the United States in 2016: An Update. Semin. Perinatol. 2021, 45, 151390. [Google Scholar] [CrossRef] [PubMed]
- Buchh, B.; Graham, N.; Harris, B.; Sims, S.; Corpuz, M.; Lantos, J.; Meadow, W. Neonatology Has Always Been a Bargain--Even When We Weren’t Very Good at It! Acta Paediatr. 2007, 96, 659–663. [Google Scholar] [CrossRef]
- Cheah, I.G.S. Economic Assessment of Neonatal Intensive Care. Transl. Pediatr. 2019, 8, 246–256. [Google Scholar] [CrossRef]
- Hoyle, E.; Spierson, H.; Cordon, D.; Brady, J. Cost of Nitric Oxide Therapy in Neonates. BMJ Paediatr. Open 2020, 4, e000776. [Google Scholar] [CrossRef]
- Levesque, B.M.; Kalish, L.A.; LaPierre, J.; Welch, M.; Porter, V. Impact of Implementing 5 Potentially Better Respiratory Practices on Neonatal Outcomes and Costs. Pediatrics 2011, 128, e218–e226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mowitz, M.E.; Zupancic, J.A.F.; Millar, D.; Kirpalani, H.; Gaulton, J.S.; Roberts, R.S.; Mao, W.; Dukhovny, D. Prospective Economic Evaluation alongside the Non-Invasive Ventilation Trial. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2017, 37, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Greenberg, R.G.; Sharma, A.; Natarajan, G.; Cotten, M.; Thomas, R.; Chawla, S. A Predictive Model for Extubation Readiness in Extremely Preterm Infants. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2019, 39, 1663–1669. [Google Scholar] [CrossRef]
- Chakraborty, M.; Watkins, W.J.; Tansey, K.; King, W.E.; Banerjee, S. Predicting Extubation Outcomes Using the Heart Rate Characteristics Index in Preterm Infants: A Cohort Study. Eur. Respir. J. 2020, 56. [Google Scholar] [CrossRef]
- Onland, W.; Debray, T.P.; Laughon, M.M.; Miedema, M.; Cools, F.; Askie, L.M.; Asselin, J.M.; Calvert, S.A.; Courtney, S.E.; Dani, C.; et al. Clinical Prediction Models for Bronchopulmonary Dysplasia: A Systematic Review and External Validation Study. BMC Pediatr. 2013, 13, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Luo, C.; Lei, M.; Shi, Z.; Cheng, X.; Wang, L.; Shen, M.; Zhang, Y.; Zhao, M.; Wang, L.; et al. Development and Validation of a Nomogram for Predicting Bronchopulmonary Dysplasia in Very-Low-Birth-Weight Infants. Front. Pediatr. 2021, 9, 648828. [Google Scholar] [CrossRef] [PubMed]
- El Faleh, I.; Faouzi, M.; Adams, M.; Gerull, R.; Chnayna, J.; Giannoni, E.; Roth-Kleiner, M. Swiss Neonatal Network Bronchopulmonary Dysplasia: A Predictive Scoring System for Very Low Birth Weight Infants. A Diagnostic Accuracy Study with Prospective Data Collection. Eur. J. Pediatr. 2021. [Google Scholar] [CrossRef]
- Partridge, E.A.; Davey, M.G.; Hornick, M.A.; McGovern, P.E.; Mejaddam, A.Y.; Vrecenak, J.D.; Mesas-Burgos, C.; Olive, A.; Caskey, R.C.; Weiland, T.R.; et al. An Extra-Uterine System to Physiologically Support the Extreme Premature Lamb. Nat. Commun. 2017, 8, 15112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Mode | Effect on Outcome | Selected References |
|---|---|---|
| Invasive Mechanical Ventilation (IMV) modes | ||
| Conventional IMV | Increased survival, ROP & neurodevelopmental impairment; unclear effect on BPD. | [1,4,11,12,22] |
| Synchronized IMV | Reduced air leaks & duration of IMV, unclear effect on BPD. | [25] |
| NAVA | None | [26,27,28] |
| PAV | Unknown | [29] |
| VTV (VCV & VG) | Reduced BPD, IMV duration, pneumothorax, death/BPD, brain damage. | [30,31,32] |
| HFV | Little effect on reducing lung damage (despite improvement of alveolar stability), better lung function at 11–14 years of age. | [33,34,35] |
| HFV + VG | Unknown | [36,37] |
| Non-Invasive Ventilation (NIV) modes | ||
| nCPAP | Reduced BPD at 36 wks, death or BPD. | [38,39] |
| Bi-level NIV | ||
| nIPPV | Reduced risk of respiratory failure and intubation. No effect on BPD. | [40,41] |
| Bi-PAP | Effects comparable to nCPAP. | [42] |
| nHFV | Reduced duration of NIV, need for IMV, incidence of IVH. | [43,44,45] |
| High flow nasal cannula (HFNC) | HFNC as primary respiratory support: Effect on mortality, BPD, similar to other NIV modes; longer respiratory support. HFNC post extubation: Reduced nose trauma and pneumothorax, longer treatment compared to nCPAP. | [46,47,48] |
| nNAVA | Similar to nCPAP | [49,50] |
| Alternative exogenous surfactant administration modes | ||
| INSURE | Increased rate of extubation failure. | [51,52] |
| LISA / MIST (+ nCPAP) | Decreased mortality or BPD at 36 wks PMA, BPD, severe IVH | [53] |
| Nebulized surfactant | Decreased intubation rate by 50% in mild-moderate respiratory distress. Ongoing metanalysis.. | [54,55,56] |
| Adjunctive treatments | ||
| iNO | Evidence does not support the use of iNO for BPD prevention. iNO could be used for severe PPHN in established BPD. | [57,58] |
| Postnatal steroids | ||
| Systemic | Early (< 4 DoL): Reduced BPD, no effect on mortality, possibly increased risk of cerebral palsy. Late (>7 DoL): Reduced extubation failure, BPD, and death or BPD, without adverse long-term neurodevelopmental outcomes. | [59,60] |
| Inhaled | Early (1–14 DoL): Compared to systemic steroids, similar effect on BPD, longer respiratory support, increased PDA rate; lower rate of asthma & similar neurodevelopment at 7 years of age. | [61] |
| Endotracheally | Endotracheal installation via surfactant is currently under investigation (PLUSS trial). | [62] |
| Oxygen | ||
| Low vs. high target | No difference in BPD. Low SpO2 (SpO2 85–89%): Increased mortality, decreased ROP. | [36,63] |
| Automated oxygen control | Contradictory results: Either no effect or decreased mortality, any ROP, ROP requiring treatment, and BPD. | [37,64,65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarafidis, K.; Chotas, W.; Agakidou, E.; Karagianni, P.; Drossou, V. The Intertemporal Role of Respiratory Support in Improving Neonatal Outcomes: A Narrative Review. Children 2021, 8, 883. https://doi.org/10.3390/children8100883
Sarafidis K, Chotas W, Agakidou E, Karagianni P, Drossou V. The Intertemporal Role of Respiratory Support in Improving Neonatal Outcomes: A Narrative Review. Children. 2021; 8(10):883. https://doi.org/10.3390/children8100883
Chicago/Turabian StyleSarafidis, Kosmas, William Chotas, Eleni Agakidou, Paraskevi Karagianni, and Vasiliki Drossou. 2021. "The Intertemporal Role of Respiratory Support in Improving Neonatal Outcomes: A Narrative Review" Children 8, no. 10: 883. https://doi.org/10.3390/children8100883
APA StyleSarafidis, K., Chotas, W., Agakidou, E., Karagianni, P., & Drossou, V. (2021). The Intertemporal Role of Respiratory Support in Improving Neonatal Outcomes: A Narrative Review. Children, 8(10), 883. https://doi.org/10.3390/children8100883







