Longitudinal Changes in Serum Creatinine Levels and Urinary Biomarkers in Late Preterm Infants during the First Postnatal Week: Association with Acute Kidney Injury and Treatment with Aminoglycoside
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Ethics
2.2. Maternal and Neonatal Demographic and Clinical Data
2.3. Measurement of Serum Creatine Levels and AKI Biomarkers
2.4. Definition of AKI
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Features of the Study Population
3.2. Comparison of Changes in Sr and Urinary Biomarkers during the First Week of Age
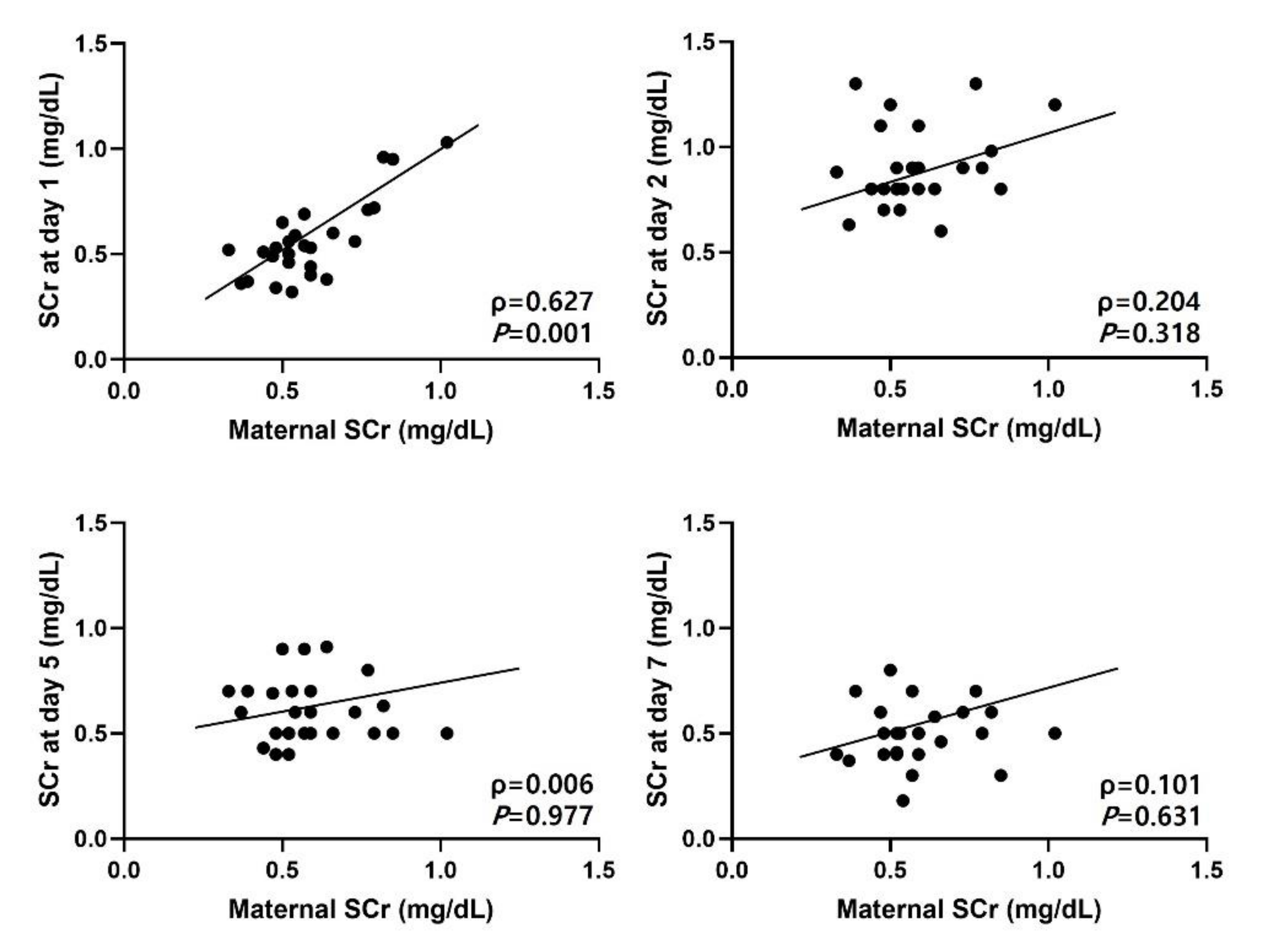
3.3. Correlation between Serum Creatinine and Urinary Biomarkers
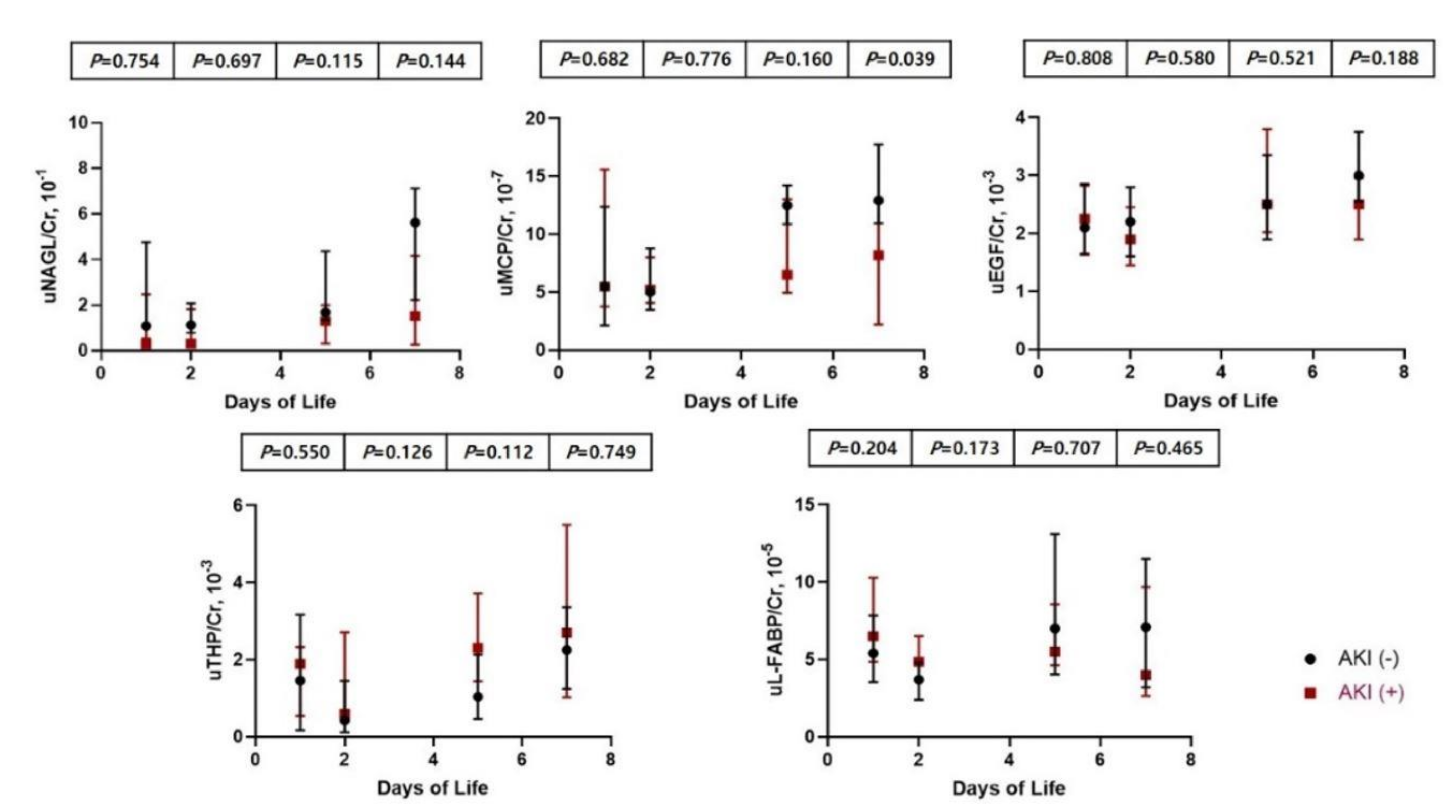
3.4. Comparison of Clinical Characteristics and Urinary Biomarkers between Infants with and without AKI
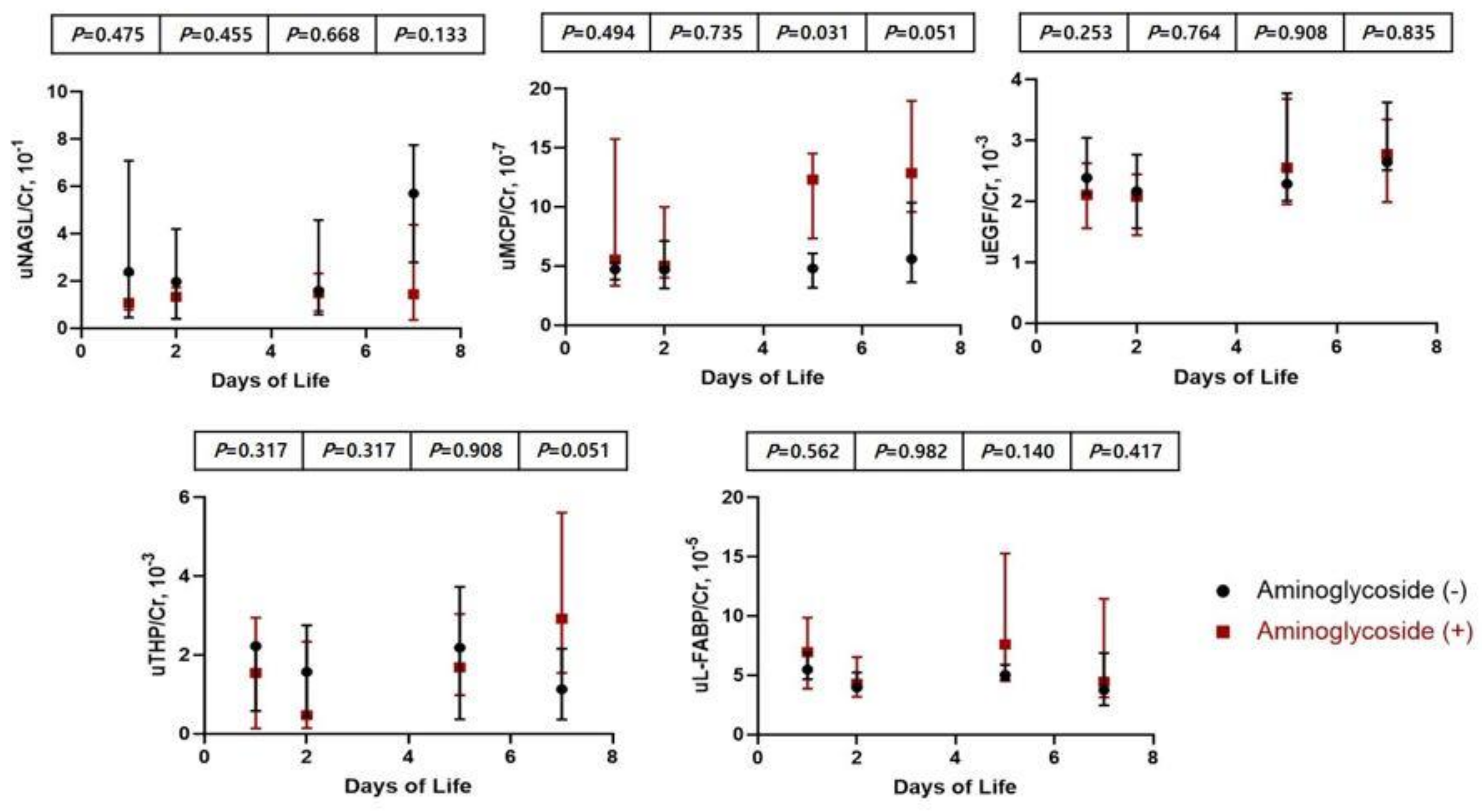
3.5. Comparison of Clinical Characteristics and Urinary Biomarkers between Infants Treated with Amionglycoside and Non-Treated Infants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Abitbol, C.L.; Rodriguez, M.M. The Long-Term Renal and Cardiovascular Consequences of Prematurity. Nat. Rev. Nephrol. 2012, 8, 265–274. [Google Scholar] [CrossRef]
- Stojanović, V.; Barišić, N.; Milanović, B.; Doronjski, A. Acute Kidney Injury in Preterm Infants Admitted to a Neonatal Intensive Care Unit. Pediatr. Nephrol. Berl. Ger. 2014, 29, 2213–2220. [Google Scholar] [CrossRef]
- Askenazi, D.J.; Griffin, R.; McGwin, G.; Carlo, W.; Ambalavanan, N. Acute Kidney Injury Is Independently Associated with Mortality in Very Low Birthweight Infants: A Matched Case-Control Analysis. Pediatr. Nephrol. Berl. Ger. 2009, 24, 991–997. [Google Scholar] [CrossRef]
- Bates, C.M.; Charlton, J.R.; Ferris, M.E.; Hildebrandt, F.; Hoshizaki, D.K.; Warady, B.A.; Moxey-Mims, M.M. Kidney Research National Dialogue Pediatric Kidney Disease: Tracking Onset and Improving Clinical Outcomes. Clin. J. Am. Soc. Nephrol. CJASN 2014, 9, 1141–1143. [Google Scholar] [CrossRef] [PubMed]
- Vachvanichsanong, P.; McNeil, E.; Dissaneevate, S.; Dissaneewate, P.; Chanvitan, P.; Janjindamai, W. Neonatal Acute Kidney Injury in a Tertiary Center in a Developing Country. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2012, 27, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Koralkar, R.; Ambalavanan, N.; Levitan, E.B.; McGwin, G.; Goldstein, S.; Askenazi, D. Acute Kidney Injury Reduces Survival in Very Low Birth Weight Infants. Pediatr. Res. 2011, 69, 354–358. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, S.J.; Antoine, D.J.; Sabbisetti, V.; Turner, M.A.; Farragher, T.; Bonventre, J.V.; Park, B.K.; Smyth, R.L.; Pirmohamed, M. Mechanism-Based Urinary Biomarkers to Identify the Potential for Aminoglycoside-Induced Nephrotoxicity in Premature Neonates: A Proof-of-Concept Study. PLoS ONE 2012, 7, e43809. [Google Scholar] [CrossRef]
- Askenazi, D.J.; Ambalavanan, N.; Goldstein, S.L. Acute Kidney Injury in Critically Ill Newborns: What Do We Know? What Do We Need to Learn? Pediatr. Nephrol. Berl. Ger. 2009, 24, 265–274. [Google Scholar] [CrossRef]
- Lunn, A.J.F.; Shaheen, I.; Watson, A.R. Acute Renal Insufficiency in the Neonatal Intensive Care Unit. Arch. Dis. Child. Fetal Neonatal Ed. 2006, 91, F388. [Google Scholar] [CrossRef]
- Cataldi, L.; Leone, R.; Moretti, U.; De Mitri, B.; Fanos, V.; Ruggeri, L.; Sabatino, G.; Torcasio, F.; Zanardo, V.; Attardo, G.; et al. Potential Risk Factors for the Development of Acute Renal Failure in Preterm Newborn Infants: A Case-Control Study. Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, F514–F519. [Google Scholar] [CrossRef]
- Elmas, A.T.; Karadag, A.; Tabel, Y.; Ozdemir, R.; Otlu, G. Analysis of Urine Biomarkers for Early Determination of Acute Kidney Injury in Non-Septic and Non-Asphyxiated Critically Ill Preterm Neonates. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2017, 30, 302–308. [Google Scholar] [CrossRef]
- Faa, G.; Gerosa, C.; Fanni, D.; Nemolato, S.; Locci, A.; Cabras, T.; Marinelli, V.; Puddu, M.; Zaffanello, M.; Monga, G.; et al. Marked Interindividual Variability in Renal Maturation of Preterm Infants: Lessons from Autopsy. J. Matern. Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2010, 23 (Suppl. 3), 129–133. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.M.; Gómez, A.H.; Abitbol, C.L.; Chandar, J.J.; Duara, S.; Zilleruelo, G.E. Histomorphometric Analysis of Postnatal Glomerulogenesis in Extremely Preterm Infants. Pediatr. Dev. Pathol. Off. J. Soc. Pediatr. Pathol. Paediatr. Pathol. Soc. 2004, 7, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, M.R.; Gubhaju, L.; Moore, L.; Kent, A.L.; Dahlstrom, J.E.; Horne, R.S.C.; Hoy, W.E.; Bertram, J.F.; Black, M.J. Accelerated Maturation and Abnormal Morphology in the Preterm Neonatal Kidney. J. Am. Soc. Nephrol. JASN 2011, 22, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.H.; Han, D.; Shin, S.H.; Kim, E.-K.; Kim, H.-S. Proteomic Identification of Early Urinary-Biomarkers of Acute Kidney Injury in Preterm Infants. Sci. Rep. 2020, 10, 4057. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M.; Brophy, P.D.; Giannone, P.J.; Joshi, M.S.; Bauer, J.A.; RamachandraRao, S. Early Urinary Biomarkers of Acute Kidney Injury in Preterm Infants. Pediatr. Res. 2016, 80, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Ivanišević, I.; Peco-Antić, A.; Vuličević, I.; Hercog, D.; Milovanović, V.; Kotur-Stevuljević, J.; Stefanović, A.; Kocev, N. L-FABP Can Be an Early Marker of Acute Kidney Injury in Children. Pediatr. Nephrol. Berl. Ger. 2013, 28, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, B.; Koralkar, R.; Griffin, R.L.; Halloran, B.; Ambalavanan, N.; Askenazi, D.J. Impact of Gestational Age, Sex, and Postnatal Age on Urine Biomarkers in Premature Neonates. Pediatr. Nephrol. Berl. Ger. 2015, 30, 2037–2044. [Google Scholar] [CrossRef]
- Beker, B.M.; Corleto, M.G.; Fieiras, C.; Musso, C.G. Novel Acute Kidney Injury Biomarkers: Their Characteristics, Utility and Concerns. Int. Urol. Nephrol. 2018, 50, 705–713. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Bellomo, R.; Kellum, J.A.; Ronco, C. Defining Acute Renal Failure: Physiological Principles. Intensive Care Med. 2004, 30, 33–37. [Google Scholar] [CrossRef]
- Askenazi, D.; Abitbol, C.; Boohaker, L.; Griffin, R.; Raina, R.; Dower, J.; Davis, T.K.; Ray, P.E.; Perazzo, S.; DeFreitas, M.; et al. Optimizing the AKI Definition during First Postnatal Week Using Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) Cohort. Pediatr. Res. 2019, 85, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Kamianowska, M.; Wasilewska, A.; Szczepański, M.; Kulikowska, E.; Bebko, B.; Koput, A. Health Term-Born Girls Had Higher Levels of Urine Neutrophil Gelatinase-Associated Lipocalin than Boys during the First Postnatal Days. Acta Paediatr. Oslo Nor. 1992 2016, 105, 1105–1108. [Google Scholar] [CrossRef]
- Huynh, T.K.; Bateman, D.A.; Parravicini, E.; Lorenz, J.M.; Nemerofsky, S.L.; Sise, M.E.; Bowman, T.M.; Polesana, E.; Barasch, J.M. Reference Values of Urinary Neutrophil Gelatinase-Associated Lipocalin in Very Low Birth Weight Infants. Pediatr. Res. 2009, 66, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Nehus, E.; Haffner, C.; Ma, Q.; Devarajan, P. Pediatric Reference Ranges for Acute Kidney Injury Biomarkers. Pediatr. Nephrol. Berl. Ger. 2015, 30, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.; Lakshmanan, J.; Hoath, S.; Fisher, D.A. Effect of Oestradiol-17 Beta on Uterine Epidermal Growth Factor Concentration in Immature Mice. Acta Endocrinol. 1984, 105, 425–428. [Google Scholar] [CrossRef]
- Perheentupa, J.; Lakshmanan, J.; Fisher, D.A. Urine and Kidney Epidermal Growth Factor: Ontogeny and Sex Difference in the Mouse. Pediatr. Res. 1985, 19, 428–432. [Google Scholar] [CrossRef]
- DeFreitas, M.J.; Seeherunvong, W.; Katsoufis, C.P.; RamachandraRao, S.; Duara, S.; Yasin, S.; Zilleruelo, G.; Rodriguez, M.M.; Abitbol, C.L. Longitudinal Patterns of Urine Biomarkers in Infants across Gestational Ages. Pediatr. Nephrol. Berl. Ger. 2016, 31, 1179–1188. [Google Scholar] [CrossRef]
- Askenazi, D.J.; Koralkar, R.; Hundley, H.E.; Montesanti, A.; Parwar, P.; Sonjara, S.; Ambalavanan, N. Urine Biomarkers Predict Acute Kidney Injury in Newborns. J. Pediatr. 2012, 161, 270–275.e1. [Google Scholar] [CrossRef]
- Uromodulin: Old Friend with New Roles in Health and Disease—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/23880785/ (accessed on 10 August 2021).
- Charlton, J.R.; Portilla, D.; Okusa, M.D. A Basic Science View of Acute Kidney Injury Biomarkers. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2014, 29, 1301–1311. [Google Scholar] [CrossRef]
- Briguori, C.; Quintavalle, C.; Donnarumma, E.; Condorelli, G. Novel Biomarkers for Contrast-Induced Acute Kidney Injury. BioMed Res. Int. 2014, 2014, 568738. [Google Scholar] [CrossRef]
- Chevalier, R.L.; Goyal, S.; Wolstenholme, J.T.; Thornhill, B.A. Obstructive Nephropathy in the Neonatal Rat Is Attenuated by Epidermal Growth Factor. Kidney Int. 1998, 54, 38–47. [Google Scholar] [CrossRef][Green Version]
- Guignard, J.P.; Drukker, A. Why Do Newborn Infants Have a High Plasma Creatinine? Pediatrics 1999, 103, e49. [Google Scholar] [CrossRef]
- Gallini, F.; Maggio, L.; Romagnoli, C.; Marrocco, G.; Tortorolo, G. Progression of Renal Function in Preterm Neonates with Gestational Age < or = 32 Weeks. Pediatr. Nephrol. Berl. Ger. 2000, 15, 119–124. [Google Scholar] [CrossRef]
- Miall, L.S.; Henderson, M.J.; Turner, A.J.; Brownlee, K.G.; Brocklebank, J.T.; Newell, S.J.; Allgar, V.L. Plasma Creatinine Rises Dramatically in the First 48 Hours of Life in Preterm Infants. Pediatrics 1999, 104, e76. [Google Scholar] [CrossRef] [PubMed]
- Barhight, M.; Altaye, M.; Gist, K.M.; Isemann, B.; Goldstein, S.L.; Akinbi, H. Nephrotoxic Medications and Associated Acute Kidney Injury in Very Low Birth Weight Infants. J. Clin. Nephrol. Res. 2017, 4, 1070. [Google Scholar] [PubMed]
- Servais, H.; Van Der Smissen, P.; Thirion, G.; Van der Essen, G.; Van Bambeke, F.; Tulkens, P.M.; Mingeot-Leclercq, M.-P. Gentamicin-Induced Apoptosis in LLC-PK1 Cells: Involvement of Lysosomes and Mitochondria. Toxicol. Appl. Pharmacol. 2005, 206, 321–333. [Google Scholar] [CrossRef]
- Giapros, V.I.; Andronikou, S.K.; Cholevas, V.I.; Papadopoulou, Z.L. Renal Function and Effect of Aminoglycoside Therapy during the First Ten Days of Life. Pediatr. Nephrol. Berl. Ger. 2003, 18, 46–52. [Google Scholar] [CrossRef]
| Variables | |
|---|---|
| Male, n (%) | 12 (40) |
| Gestational age, wks | 34.2 (34.0–35.3) |
| Birth weight, g | 2130 (1960–2300) |
| Cesarean section | 20 (67) |
| Apgar score | |
| 1 min | 7 (7–8) |
| 5 min | 9 (8–9) |
| Weight loss, % | −1.0 (−2.4–1.0) |
| AKI | 18 (60) |
| Oliguria | 0 |
| PPV | 20 (67) |
| Oligohydramnios | 2 (6.7) |
| Medication history | |
| Aminoglycoside | 20 (67) |
| Steroid | 0 |
| Ibuprofen | 0 |
| Diuretics | 0 |
| Inotrope | 0 |
| Maternal characteristics | |
| Diabetes | 7 (23) |
| PIH | 3 (10) |
| PROM | 11 (37) |
| Steroid | 9 (30) |
| Antibiotics | 8 (27) |
| BUN, mg/dL | 6.9 (5.9–9.6) |
| Creatinine, mg/dL | 0.56 (0.49–0.66) |
| 1st | 2nd | 5th | 7th | p-Value | |
|---|---|---|---|---|---|
| Maternal SCr, mg/dL | 0.56 (0.49–0.66) | 0.56 (0.49–0.66) | 0.56 (0.49–0.66) | 0.56 (0.49–0.66) | - |
| SCr, mg/dL | 0.53 (0.46–0.69) | 0.88 (0.80–0.98) | 0.60 (0.50–0.70) | 0.50 (0.40–0.60) | <0.001 |
| uNGAL/Cr, 10−1 | 1.09 (0.55–2.47) | 1.34 (0.38–1.91) | 1.49 (0.63–2.95) | 2.21 (0.39–6.57) | 0.497 |
| uMCP-1/Cr, 10−7 | 5.47 (3.50–15.27) | 5.01 (3.92–9.22) | 9.18 (5.56–13.64) | 10.60 (4.11–17.01) | 0.116 |
| uEGF/Cr, 10−3 | 2.19 (1.62–2.88) | 2.13 (1.45–2.62) | 2.49 (1.96–3.78) | 2.73 (2.00–3.63) | 0.042 |
| uTHP/Cr, 10−3 | 1.69 (0.22–2.77) | 0.54 (0.20–2.50) | 1.76 (0.81–3.72) | 2.39 (0.99–4.31) | 0.023 |
| uL-FABP/Cr, 10−5 | 6.07 (4.21–9.12) | 4.07 (3.48–6.26) | 5.89 (4.58–10.47) | 4.10 (3.07–11.10) | 0.122 |
| AKI (n = 18) | Non-AKI (n = 12) | p-Value | |
|---|---|---|---|
| Male | 7 (39) | 5 (42) | 0.514 |
| Gestational age, weeks | 34.1 (34.0–34.3) | 35.1 (34.4–35.5) | 0.016 |
| Birth weight, g | 1990 (1923–2190) | 2240 (2180–2450) | 0.016 |
| Weight loss, % | −0.65 (−1.55–0.75) | −1.0 (−3.6–0) | 0.521 |
| Cesarean section | 12 (67) | 8 (67) | 0.534 |
| Apgar score at 1 min | 7.0 (7.0–8.0) | 7.5 (7.0–8.0) | 0.588 |
| Apgar score at 5 min | 9 (8.0–9.0) | 9 (9.0–9.0) | 0.265 |
| PPV, n (%) | 13 (72) | 8 (67) | 0.659 |
| Aminoglycoside, n (%) | 12 (67) | 8 (67) | 0.592 |
| SCr at day 1, mg/dL | 0.50 (0.41–0.54) | 0.72 (0.60–0.96) | 0.001 |
| SCr at day 2, mg/dL | 0.89 (0.80–1.05) | 0.80 (0.80–0.94) | 0.492 |
| SCr at day 5, mg/dL | 0.65 (0.50–0.70) | 0.50 (0.45–0.55) | 0.014 |
| SCr at day 7, mg/dL | 0.50 (0.41–0.66) | 0.37 (0.30–0.50) | 0.007 |
| Maternal Characteristics | |||
| SCr, mg/dL | 0.52 (0.48–0.59) | 0.66 (0.54–0.82) | 0.085 |
| Diabetes, n (%) | 4 (22) | 3 (25) | 0.547 |
| PIH, n (%) | 1 (6) | 2 (17) | 0.316 |
| PROM, n (%) | 8 (44) | 3 (25) | 0.449 |
| Steroid, n (%) | 7 (39) | 2 (17) | 0.412 |
| Antibiotics, n (%) | 6 (33) | 2 (17) | 0.671 |
| Aminoglycoside (n = 20) | Non-Aminoglycoside (n = 10) | p-Value | |
|---|---|---|---|
| Male, n (%) | 9 (45) | 3 (30) | 0.694 |
| Gestational age, weeks | 34.2 (34.1–35.2) | 34.3 (34.0–35.3) | 0.871 |
| Birth weight, g | 2115 (1960–2320) | 2190 (1930–2230) | 0.729 |
| Weight loss, % | −1.0 (−2.4–1.0) | −1.0 (−2.4–−0.2) | 0.501 |
| C-sec, n (%) | 15 (75) | 5 (50%) | 0.339 |
| Aminoglycoside (n = 20) | Non-aminoglycoside (n = 10) | p-value | |
| Apgar score at 1 min | 7 (7–8) | 8 (7–8) | 0.923 |
| Apgar score at 5 min | 9 (8–9) | 9 (8–9) | 0.885 |
| Positive pressure ventilation, n (%) | 17 (85) | 4 (40) | 0.067 |
| AKI, n (%) | 12 (60) | 6 (60) | 0.412 |
| SCr at day 1, mg/dL | 0.56 (0.46–0.72) | 0.52 (0.46–0.54) | 0.390 |
| SCr at day 2, mg/dL | 0.85 (0.80–0.99) | 0.88 (0.80–0.90) | 0.729 |
| SCr at day 5, mg/dL | 0.5 (0.49–0.61) | 0.69 (0.50–0.70) | 0.085 |
| SCr at day 7, mg/dL | 0.5 (0.4–0.55) | 0.50 (0.40–0.60) | 1.000 |
| Maternal Characteristics | |||
| SCr, mg/dL | 0.57 (0.50–0.73) | 0.54 (0.47–0.59) | 0.458 |
| GDM, n (%) | 4 (20) | 3 (30) | 1.000 |
| PIH, n (%) | 2 (10) | 1 (10) | 1.000 |
| PROM, n (%) | 6 (30) | 5 (50) | 0.237 |
| Steroid, n (%) | 6 (30) | 3 (30) | 1.000 |
| Antibiotics, n (%) | 6 (30) | 2 (20) | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-Y.; Moon, J.-E.; Park, S.-H. Longitudinal Changes in Serum Creatinine Levels and Urinary Biomarkers in Late Preterm Infants during the First Postnatal Week: Association with Acute Kidney Injury and Treatment with Aminoglycoside. Children 2021, 8, 896. https://doi.org/10.3390/children8100896
Lee S-Y, Moon J-E, Park S-H. Longitudinal Changes in Serum Creatinine Levels and Urinary Biomarkers in Late Preterm Infants during the First Postnatal Week: Association with Acute Kidney Injury and Treatment with Aminoglycoside. Children. 2021; 8(10):896. https://doi.org/10.3390/children8100896
Chicago/Turabian StyleLee, Sang-Yoon, Jung-Eun Moon, and Sook-Hyun Park. 2021. "Longitudinal Changes in Serum Creatinine Levels and Urinary Biomarkers in Late Preterm Infants during the First Postnatal Week: Association with Acute Kidney Injury and Treatment with Aminoglycoside" Children 8, no. 10: 896. https://doi.org/10.3390/children8100896
APA StyleLee, S.-Y., Moon, J.-E., & Park, S.-H. (2021). Longitudinal Changes in Serum Creatinine Levels and Urinary Biomarkers in Late Preterm Infants during the First Postnatal Week: Association with Acute Kidney Injury and Treatment with Aminoglycoside. Children, 8(10), 896. https://doi.org/10.3390/children8100896






