Complex Evaluation of Tissue Factors in Pediatric Cholesteatoma
Abstract
:1. Introduction
2. Materials and Methods
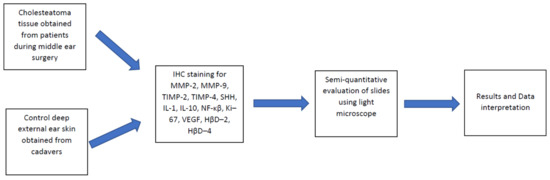
2.1. Tissue Samples
2.2. Immunohistochemical Analysis
2.3. Statistical Analysis
3. Results
3.1. Findings of Routine Histological Analysis
3.2. Immunohistochemistry Findings for Tissue Remodeling Factors
3.3. Immunohistochemistry Findings for Shh Gene Protein
3.4. Immunohistochemistry Findings for Pro- and Anti-Inflammatory Cytokines
3.5. Immunohistochemistry Findings for Cellular Proliferation Markers
3.6. Immunohistochemistry Findings for Angiogenetic Factor
3.7. Immunohistochemistry Findings for Human Beta Defensins
3.8. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhutta, M.F.; Williamson, I.G.; Sudhoff, H.H. Cholesteatom [Cholesteatoma]. Praxis 2011, 100, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, E.; Wagner, M.; Bernal-Sprekelsen, M.; Ebmeyer, J.; Dazert, S.; Hildmann, H.; Sudhoff, H. Etiopathogenesis of cholesteatoma. Eur. Arch Otorhinolaryngol. 2004, 261, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Yabluchanskiy, A.; Ma, Y.; Iyer, R.P.; Hall, M.E.; Lindsey, M.L. Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology 2013, 28, 391–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frangogiannis, N.G.; Smith, C.W.; Entman, M.L. The inflammatory response in myocardial infarction. Cardiovas. Res. 2002, 53, 31–47. [Google Scholar] [CrossRef]
- Morales, D.S.R.; de Oliveira Penido, N.; da Silva, I.D.C.G.; Stávale, J.N.; Guilherme, A.; Fukuda, Y. Matrix Metalloproteinase 2: An important genetic marker for cholesteatomas. Braz. J. Otorhinolaryngol. 2007, 73, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Hemler, M.E. Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res. 2001, 61, 2276–2281. [Google Scholar]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [Green Version]
- Olszewska, E.; Matulka, M.; Mroczko, B.; Pryczynicz, A.; Kemona, A.; Szmitkowski, M.; Mierzwinski, J.; Pietrewicz, T. Diagnostic value of matrix metalloproteinase 9 and tissue inhibitor of matrix metalloproteinases 1 in cholesteatoma. Histol. Histopathol. 2016, 31, 307–315. [Google Scholar] [CrossRef]
- Schönermark, M.; Mester, B.; Kempf, H.G.; Bläser, J.; Tschesche, H.; Lenarz, T. Expression of matrix-metalloproteinases and their inhibitors in human cholesteatomas. Acta Oto-Laryngol. 1996, 116, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Kaya, İ.; Avcı, Ç.B.; Şahin, F.F.; Özateş, N.P.; Sezgin, B.; Kurt, C.Ç.; Bilgen, C.; Kirazlı, T. Evaluation of significant gene expression changes in congenital and acquired cholesteatoma. Mol. Biol. Rep. 2020, 47, 6127–6133. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.E.; Wang, M.; Greene, J.; Su, J.; Ullrich, S.; Li, H.; Sheng, S.; Alexander, P.; Sang, Q.A.; Shi, Y.E. Preparation and characterization of recombinant tissue inhibitor of metalloproteinase 4 (TIMP-4). J. Biol. Chem. 1997, 272, 20479–20483. [Google Scholar] [CrossRef] [Green Version]
- Ahlgren, S.C.; Bronner-Fraser, M. Inhibition of sonic hedgehog signaling in vivo results in craniofacial neural crest cell death. Curr. Biol. CB 1999, 9, 1304–1314. [Google Scholar] [CrossRef] [Green Version]
- Brito, J.M.; Teillet, M.A.; Le Douarin, N.M. An early role for sonic hedgehog from foregut endoderm in jaw development: Ensuring neural crest cell survival. Proc. Natl. Acad. Sci. USA 2006, 103, 11607–11612. [Google Scholar] [CrossRef] [Green Version]
- Brito, J.M.; Teillet, M.A.; Le Douarin, N.M. Induction of mirror-image supernumerary jaws in chicken mandibular mesenchyme by Sonic Hedgehog-producing cells. Development 2008, 135, 2311–2319. [Google Scholar] [CrossRef] [Green Version]
- Wright, C.G. Development of the human external ear. J. Am. Acad. Audiol. 1997, 8, 379–382. [Google Scholar] [PubMed]
- Lange, W. Tief eingezogene Membrana flaccida und Cholesteatom. Ztschr. F. Hals Nasen-U. Ohrenh. 1932, 30, 575. [Google Scholar]
- Kuo, C.L. Etiopathogenesis of acquired cholesteatoma: Prominent theories and recent advances in biomolecular research. Laryngoscope 2015, 125, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Xiang, Y.; Wang, X.; Ren, H.; Yin, T.; Ren, J.; Liu, W. Acquired cholesteatoma epithelial hyperproliferation: Roles of cell proliferation signal pathways. Laryngoscope 2016, 126, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Fujikado, N.; Manaka, H.; Yasuda, H.; Iwakura, Y. IL-1 plays an important role in the bone metabolism under physiological conditions. Int. Immunol. 2010, 22, 805–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzun, T.; Çaklı, H.; Coşan, D.T.; İncesulu, Ş.A.; Kaya, E.; Çalış, İ.U.; Yıldız, E. In vitro study on immune response modifiers as novel medical treatment options for cholesteatoma. Int. J. Pediatr. Otorhinolaryngol. 2021, 145, 110743. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Hamajima, Y.; Zhang, Q.; Lin, J. Identification of Id1 in acquired middle ear cholesteatoma. Arch. Otolaryngol. Head Neck Surg. 2008, 134, 306–310. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Qin, Z.B. Inflammation-induced miR-802 promotes cell proliferation in cholesteatoma. Biotechnol. Lett. 2014, 36, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Mallet, Y.; Nouwen, J.; Lecomte-Houcke, M.; Desaulty, A. Aggressiveness and quantification of epithelial proliferation of middle ear cholesteatoma by MIB1. Laryngoscope 2003, 113, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Bujía, J.; Kim, C.; Holly, A.; Sudhoff, H.; Ostos, P.; Kastenbauer, E. Epidermal growth factor receptor (EGF-R) in human middle ear cholesteatoma: An analysis of protein production and gene expression. Am. J. Otol. 1996, 17, 203–206. [Google Scholar]
- Hamajima, Y.; Komori, M.; Preciado, D.A.; Choo, D.I.; Moribe, K.; Murakami, S.; Ondrey, F.G.; Lin, J. The role of inhibitor of DNA-binding (Id1) in hyperproliferation of keratinocytes: The pathological basis for middle ear cholesteatoma from chronic otitis media. Cell Prolif. 2010, 43, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Hasina, R.; Whipple, M.E.; Martin, L.E.; Kuo, W.P.; Ohno-Machado, L.; Lingen, M.W. Angiogenic heterogeneity in head and neck squamous cell carcinoma: Biological and therapeutic implications. Lab. Investig. A J. Tech. Methods Pathol. 2008, 88, 342–353. [Google Scholar] [CrossRef] [Green Version]
- Fukudome, S.; Wang, C.; Hamajima, Y.; Ye, S.; Zheng, Y.; Narita, N.; Sunaga, H.; Fujieda, S.; Hu, X.; Feng, L.; et al. Regulation of the angiogenesis of acquired middle ear cholesteatomas by inhibitor of DNA binding transcription factor. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 273–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudhoff, H.; Dazert, S.; Gonzales, A.M.; Borkowski, G.; Park, S.Y.; Baird, A.; Hildmann, H.; Ryan, A.F. Angiogenesis and angiogenic growth factors in middle ear cholesteatoma. Am. J. Otol. 2000, 21, 793–798. [Google Scholar]
- Frank, S.; Hübner, G.; Breier, G.; Longaker, M.T.; Greenhalgh, D.G.; Werner, S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J. Biol. Chem. 1995, 270, 12607–12613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottaviani, F.; Neglia, C.B.; Berti, E. Cytokines and adhesion molecules in middle ear cholesteatoma. A role in epithelial growth? Acta Oto-Laryngol. 1999, 119, 462–467. [Google Scholar] [CrossRef]
- Milewski, C. Die Rolle des Perimatrixfibroblasten bei der Entstehung des erworbenen Mittelohrcholesteatoms. Eine Hypothese [Role of perimatrix fibroblasts in development of acquired middle ear cholesteatoma. A hypothesis]. HNO 1998, 46, 494–501. [Google Scholar] [CrossRef]
- Brook, I. Aerobic and anaerobic bacteriology of cholesteatoma. Laryngoscope 1981, 91, 250–253. [Google Scholar] [CrossRef]
- Harder, J.; Meyer-Hoffert, U.; Teran, L.M.; Schwichtenberg, L.; Bartels, J.; Maune, S.; Schröder, J.M. Mucoid Pseudomonas aeruginosa, TNF-alpha, and IL-1beta, but not IL-6, induce human beta-defensin-2 in respiratory epithelia. Am. J. Respir. Cell Mol. Biol. 2000, 22, 714–721. [Google Scholar] [CrossRef]
- Smiley, A.K.; Gardner, J.; Klingenberg, J.M.; Neely, A.N.; Supp, D.M. Expression of human beta defensin 4 in genetically modified keratinocytes enhances antimicrobial activity. J. Burn. Care Res. Off. Publ. Am. Burn. Assoc. 2007, 28, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Moon, S.K.; Choung, Y.H.; Choi, H.S. Expression of beta-defensins in human middle ear cholesteatoma. Acta Oto-Laryngol. 2003, 123, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Chessa, C.; Bodet, C.; Jousselin, C.; Wehbe, M.; Lévêque, N.; Garcia, M. Antiviral and Immunomodulatory Properties of Antimicrobial Peptides Produced by Human Keratinocytes. Front. Microbiol. 2020, 11, 1155. [Google Scholar] [CrossRef]
- Pilmane, M.; Rumba, I.; Sundler, F.; Luts, A. Patterns of distribution and occurrence of neuroendocrine elements in lungs of humans with chronic lung disease. Proc. Latv. Acad. Sci. 1998, 52, 144–152. [Google Scholar]
- Dambergs, K.; Sumeraga, G.; Pilmane, M. Proliferation Markers, Remodeling Factors, Cytokines, Antimicrobial Peptides and Gene Proteins in Cholesteatoma. Available online: https://biomedres.us/fulltexts/BJSTR.MS.ID.004039.php (accessed on 1 September 2021).
- Banerjee, A.R.; James, R.; Narula, A.A. Matrix metalloproteinase-2 and matrix metalloproteinase-9 in cholesteatoma and deep meatal skin. Clin. Otolaryngol. Allied Sci. 1998, 23, 345–347. [Google Scholar] [CrossRef]
- Rezende, C.E.; Souto, R.P.; Rapoport, P.B.; de Campos, L.; Generato, M.B. Cholesteatoma gene expression of matrix metalloproteinases and their inhibitors by RT-PCR. Braz. J. Otorhinolaryngol. 2012, 78, 116–121. [Google Scholar] [CrossRef] [Green Version]
- Olszewska, E.; Chodynicki, S.; Chyczewski, L. Znaczenie angiogenezy w patogenezie perlaka ucha środkowego u dorosłych [Role of angiogenesis in the pathogenesis of cholesteatoma in adults]. Otolaryngol Pol. 2004, 58, 559–563. [Google Scholar] [PubMed]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [Green Version]
- Dworkin, S.; Boglev, Y.; Owens, H.; Goldie, S.J. The role of sonic hedgehog in craniofacial patterning, morphogenesis and cranial neural crest survival. J. Dev. Biol. 2016, 4, 24. [Google Scholar] [CrossRef] [Green Version]
- Yetiser, S.; Satar, B.; Aydin, N. Expression of epidermal growth factor, tumor necrosis factor-alpha, and interleukin-1alpha in chronic otitis media with or without cholesteatoma. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2002, 23, 647–652. [Google Scholar] [CrossRef]
- Kuczkowski, J.; Sakowicz-Burkiewicz, M.; Iżycka-Świeszewska, E.; Mikaszewski, B.; Pawełczyk, T. Expression of tumor necrosis factor-α, interleukin-1α, interleukin-6 and interleukin-10 in chronic otitis media with bone osteolysis. ORL J. Oto-Rhino-Laryngol. Its Relat. Spec. 2011, 73, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Lee, S.H.; Park, C.W.; Kim, K.R.; Tae, K.; Kang, S.H.; Oh, Y.H.; Pyo, J.Y. Expression of apoptotic vs antiapoptotic proteins in middle ear cholesteatoma. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 2015, 153, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.A.; Nakata, S.; Shiogama, K.; Suzuki, K.; Sayed, R.H.; Nishimura, Y.; Iwata, N.; Sakurai, K.; Badawy, B.S.; Inada, K.I.; et al. Cytokeratin 13, cytokeratin 17, and ki-67 expression in human acquired cholesteatoma and their correlation with its destructive capacity. Clin. Exp. Otorhinolaryngol. 2017, 10, 213–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.H.; Lim, H.J.; Kim, Y.J.; Kim, S.W.; Kim, Y.S.; Tian, C.; Park, K.; Park, T.J.; Choung, Y.H. The oncoprotein, gankyrin, is up-regulated in middle ear cholesteatoma. Acta Oto-Laryngol. 2014, 134, 238–243. [Google Scholar] [CrossRef]
- Kuczkowski, J.; Pawelczyk, T.; Bakowska, A.; Narozny, W.; Mikaszewski, B. Expression patterns of Ki-67 and telomerase activity in middle ear cholesteatoma. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2007, 28, 204–207. [Google Scholar] [CrossRef]
- Chae, S.W.; Song, J.J.; Suh, H.K.; Jung, H.H.; Lim, H.H.; Hwang, S.J. Expression patterns of p27Kip1 and Ki-67 in cholesteatoma epithelium. Laryngoscope 2000, 110, 1898–1901. [Google Scholar] [CrossRef]
- Soliman, N.A.; Yussif, S.M. Ki-67 as a prognostic marker according to breast cancer molecular subtype. Cancer Biol. Med. 2016, 13, 496–504. [Google Scholar] [CrossRef] [Green Version]
- Mercadante, A.A.; Kasi, A. Genetics, Cancer Cell Cycle Phases; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Byun, J.Y.; Yune, T.Y.; Lee, J.Y.; Yeo, S.G.; Park, M.S. Expression of CYLD and NF-kappaB in human cholesteatoma epithelium. Mediat. Inflamm. 2010, 2010, 796315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Yin, T.; Ren, J.; Li, L.; Xiao, Z.; Chen, X.; Xie, D. Activation of the EGFR/Akt/NF-κB/cyclinD1 survival signaling pathway in human cholesteatoma epithelium. Eur. Arch. Oto-Rhino-Laryngol. Off. J. Eur. Fed. Oto-Rhino-Laryngol. Soc. (EUFOS) Affil. Ger. Soc. Oto-Rhino-Laryngol. Head Neck Surg. 2014, 271, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Chae, S.W.; Woo, J.S.; Lee, H.M.; Jung, H.H.; Hwang, S.J. Differential expression of human beta defensin 2 and human beta defensin 3 in human middle ear cholesteatoma. Ann. Otol. Rhinol. Laryngol. 2007, 116, 235–240. [Google Scholar] [CrossRef]
- Varoga, D.; Tohidnezhad, M.; Paulsen, F.; Wruck, C.J.; Brandenburg, L.; Mentlein, R.; Lippross, S.; Hassenpflug, J.; Besch, L.; Müller, M.; et al. The role of human beta-defensin-2 in bone. J. Anat. 2008, 213, 749–757. [Google Scholar] [CrossRef]
- Wehkamp, K.; Schwichtenberg, L.; Schröder, J.M.; Harder, J. Pseudomonas aeruginosa- and IL-1beta-mediated induction of human beta-defensin-2 in keratinocytes is controlled by NF-kappaB and AP-1. J. Investig. Dermatol. 2006, 126, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.K.; Lee, H.Y.; Li, J.D.; Nagura, M.; Kang, S.H.; Chun, Y.M.; Linthicum, F.H.; Ganz, T.; Andalibi, A.; Lim, D.J. Activation of a Src-dependent Raf-MEK1/2-ERK signaling pathway is required for IL-1alpha-induced upregulation of beta-defensin 2 in human middle ear epithelial cells. Biochim. Et Biophys. Acta 2002, 1590, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Urík, M.; Hurník, P.; Žiak, D.; Machač, J.; Šlapák, I.; Motyka, O.; Jabandžiev, P. Immunohistochemical analysis of retraction pocket pars tensa of tympanic membrane in children. Int. J. Pediatric Otorhinolaryngol. 2019, 122, 111–116. [Google Scholar] [CrossRef] [PubMed]












| Detected Factor | Mann–Whitney U Test | Z-Score | p-Value |
|---|---|---|---|
| Shh perimatrix and Shh control connective tissue | 25,500 | −3180 | 0.001 |
| NF-κβ matrix and NF-κβ control epithelium | 21,500 | −3332 | 0.001 |
| NF-κβ perimatrix and NF-κβ control connective tissue | 40,000 | −2451 | 0.017 |
| HβD-2 perimatrix and HβD-2 control connective tissue | 22,500 | −3326 | 0.001 |
| HβD-4 matrix and HβD-4 control epithelium | 167,500 | 4001 | 0.000 |
| Markers | MMP-2 M | MMP-2 P | MMP-9 M | MMP-9 P | TIMP-2 M | TIMP-2 P | TIMP-4 M | TIMP-4 P | Shh M | Shh P | IL-1 M | IL-1 P | IL-10 M | IL-10 P | NF-κβ M | NF-κβ P | Ki-67 M | Ki-67 P | VEGF M | VEGF P | HβD2 M | HβD2 P | HβD4 M | HβD4 P | |
| MMP-2 M | Rs p | ||||||||||||||||||||||||
| MMP-2 P | Rs p | 0.824 ** 0.000 | |||||||||||||||||||||||
| MMP-9 M | Rs p | 0.265 0.320 | 0.487 0.056 | ||||||||||||||||||||||
| MMP-9 P | Rs p | 0.212 0.430 | 0.471 0.066 | 0.701 ** 0.002 | |||||||||||||||||||||
| TIMP-2 M | Rs p | 0.710 ** 0.002 | 0.615 * 0.011 | 0.465 0.070 | 0.363 0.167 | ||||||||||||||||||||
| TIMP-2 P | Rs p | 0.612 * 0.012 | 0.793 ** 0.000 | 0.587 * 0.017 | 0.678 ** 0.004 | 0.718 ** 0.002 | |||||||||||||||||||
| TIMP-4 M | Rs p | 0.366 0.163 | 0.513 * 0.042 | 0.547 * 0.028 | 0.371 0.157 | 0.145 0.592 | 0.370 0.158 | ||||||||||||||||||
| TIMP-4 P | Rs p | 0.197 0.466 | 0.354 0.179 | 0.286 0.282 | 0.198 0.463 | −0.099 0.715 | 0.134 0.620 | 0.747 ** 0.001 | |||||||||||||||||
| Shh M | Rs p | 0.337 0.202 | 0.435 0.093 | 0.493 0.053 | 0.447 0.083 | 0.248 0.354 | 0.440 0.088 | 0.745 ** 0.001 | 0.463 0.071 | ||||||||||||||||
| Shh P | Rs p | 0.462 0.072 | 0.535 * 0.033 | 0.494 0.052 | 0.383 0.143 | 0.488 0.055 | 0.610 * 0.012 | 0.538 * 0.032 | 0.366 0.164 | 0.845 ** 0.000 | |||||||||||||||
| IL-1 M | Rs p | 0.327 0.216 | 0.288 0.280 | 0.195 0.468 | 0.170 0.530 | 0.187 0.488 | 0.199 0.460 | 0.702 ** 0.002 | 0.594 * 0.015 | 0.576 * 0.020 | 0.286 0.284 | ||||||||||||||
| IL-1 P | Rs p | −0.040 0.883 | 0.237 0.376 | 0.597 * 0.015 | 0.646 ** 0.007 | 0.176 0.514 | 0.454 0.077 | 0.493 0.052 | 0.487 0.056 | 0.323 0.222 | 0.230 0.391 | 0.318 0.229 | |||||||||||||
| IL-10 M | Rs p | 0.308 0.246 | 0.302 0.255 | 0.006 0.982 | 0.047 0.864 | 0.320 0.226 | 0.290 0.276 | 0.269 0.313 | 0.368 0.161 | 0.599 * 0.014 | 0.580 * 0.019 | 0.543 * 0.030 | 0.177 0.513 | ||||||||||||
| IL-10 P | Rs p | 0.118 0.663 | 0.252 0.346 | 0.255 0.341 | 0.248 0.354 | 0.074 0.784 | 0.258 0.336 | 0.782 ** 0.000 | 0.811 ** 0.000 | 0.588 * 0.016 | 0.446 0.083 | 0.707 ** 0.002 | 0.594 * 0.015 | 0.466 0.069 | |||||||||||
| NF-κβ M | Rs p | 0.312 0.239 | 0.387 0.139 | 0.334 0.206 | 0.382 0.144 | 0.133 0.624 | 0.189 0.483 | 0.700 ** 0.003 | 0.663 ** 0.005 | 0.734 ** 0.001 | 0.486 0.056 | 0.768 ** 0.001 | 0.246 0.358 | 0.526 * 0.036 | 0.640 ** 0.008 | ||||||||||
| NF-κβ P | Rs p | −0.167 0.535 | 0.112 0.680 | 0.328 0.215 | 0.442 0.087 | 0.143 0.598 | 0.350 0.183 | 0.258 0.334 | 0.254 0.343 | 0.509 * 0.044 | 0.502 * 0.048 | 0.291 0.274 | 0.370 0.159 | 0.403 0.122 | 0.432 0.095 | 0.552 * 0.027 | |||||||||
| Ki-67 M | Rs p | 0.112 0.679 | 0.118 0.664 | 0.243 0.364 | 0.043 0.873 | 0.141 0.603 | 0.184 0.495 | 0.434 0.093 | 0.455 0.077 | 0.706 ** 0.002 | 0.712 ** 0.002 | 0.507 * 0.045 | 0.246 0.359 | 0.641 ** 0.007 | 0.533 * 0.033 | 0.566 * 0.022 | 0.631 ** 0.009 | ||||||||
| Ki-67 P | Rs p | −0.095 0.727 | 0.028 0.919 | 0.095 0.727 | 0.230 0.390 | −0.144 0.595 | 0.150 0.580 | 0.404 0.121 | 0.253 0.345 | 0.671 ** 0.004 | 0.487 0.056 | 0.333 0.208 | 0.250 0.350 | 0.376 0.152 | 0.439 0.089 | 0.546 * 0.029 | 0.720 ** 0.002 | 0.687 ** 0.003 | |||||||
| VEGF M | Rs p | 0.019 0.944 | 0.344 0.193 | 0.562 * 0.023 | 0.657 ** 0.006 | 0.235 0.380 | 0.435 0.092 | 0.445 0.084 | 0.380 0.146 | 0.528 * 0.036 | 0.487 0.056 | 0.225 0.402 | 0.398 0.127 | 0.230 0.392 | 0.497 0.050 | 0.623 ** 0.010 | 0.760 ** 0.001 | 0.273 0.305 | 0.424 0.101 | ||||||
| VEGF P | Rs p | 0.296 0.266 | 0.618 * 0.011 | 0.684 ** 0.004 | 0.713 ** 0.002 | 0.305 0.250 | 0.632 ** 0.009 | 0.764 ** 0.001 | 0.470 0.066 | 0.573 * 0.020 | 0.494 0.052 | 0.407 0.118 | 0.563 * 0.023 | 0.053 0.845 | 0.508 * 0.045 | 0.571 * 0.021 | 0.526 * 0.036 | 0.234 0.384 | 0.403 0.122 | 0.675 ** 0.004 | |||||
| HβD-2 M | Rs p | −0.048 0.860 | 0.045 0.868 | 0.202 0.453 | 0.262 0.327 | 0.037 0.890 | 0.085 0.754 | 0.536 * 0.032 | 0.437 0.091 | 0.675 ** 0.004 | 0.375 0.152 | 0.671 ** 0.004 | 0.364 0.166 | 0.653 ** 0.006 | 0.604 * 0.013 | 0.738 ** 0.001 | 0.578 * 0.019 | 0.501 * 0.048 | 0.616 * 0.011 | 0.516 * 0.041 | 0.386 0.140 | ||||
| HβD-2 P | Rs p | 0.351 0.182 | 0.390 0.135 | 0.171 0.525 | 0.216 0.421 | 0.384 0.142 | 0.511 * 0.043 | 0.448 0.081 | 0.261 0.329 | 0.680 ** 0.004 | 0.560 * 0.024 | 0.664 ** 0.005 | 0.350 0.184 | 0.847 ** 0.000 | 0.499 * 0.049 | 0.436 0.091 | 0.347 0.188 | 0.552 * 0.027 | 0.419 0.106 | 0.165 0.540 | 0.265 0.321 | 0.649 ** 0.007 | |||
| HβD-4 M | Rs p | 0.426 0.100 | 0.373 0.155 | 0.142 0.601 | 0.301 0.258 | 0.441 0.088 | 0.591 * 0.016 | 0.052 0.847 | 0.200 0.457 | 0.356 0.176 | 0.650 ** 0.006 | 0.006 0.983 | 0.233 0.386 | 0.473 0.064 | 0.219 0.415 | 0.148 0.585 | 0.401 0.124 | 0.489 0.055 | 0.387 0.138 | 0.228 0.396 | 0.155 0.565 | 0.118 0.662 | 0.366 0.163 | ||
| HβD-4 P | Rs p | 0.426 0.100 | 0.373 0.155 | 0.142 0.601 | 0.301 0.258 | 0.441 0.088 | 0.591 * 0.016 | 0.052 0.847 | 0.200 0.457 | 0.356 0.176 | 0.650 ** 0.006 | 0.006 0.983 | 0.233 0.386 | 0.473 0.064 | 0.219 0.415 | 0.148 0.585 | 0.401 0.124 | 0.489 0.055 | 0.387 0.138 | 0.228 0.396 | 0.155 0.565 | 0.118 0.662 | 0.366 0.163 | 0.426 0.100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dambergs, K.; Sumeraga, G.; Pilmane, M. Complex Evaluation of Tissue Factors in Pediatric Cholesteatoma. Children 2021, 8, 926. https://doi.org/10.3390/children8100926
Dambergs K, Sumeraga G, Pilmane M. Complex Evaluation of Tissue Factors in Pediatric Cholesteatoma. Children. 2021; 8(10):926. https://doi.org/10.3390/children8100926
Chicago/Turabian StyleDambergs, Kristaps, Gunta Sumeraga, and Māra Pilmane. 2021. "Complex Evaluation of Tissue Factors in Pediatric Cholesteatoma" Children 8, no. 10: 926. https://doi.org/10.3390/children8100926
APA StyleDambergs, K., Sumeraga, G., & Pilmane, M. (2021). Complex Evaluation of Tissue Factors in Pediatric Cholesteatoma. Children, 8(10), 926. https://doi.org/10.3390/children8100926