The Type of Conservative Management Could Be Related to the Strength of the Inspiratory Muscles of Adolescents with Idiopathic Scoliosis—A Case Series
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
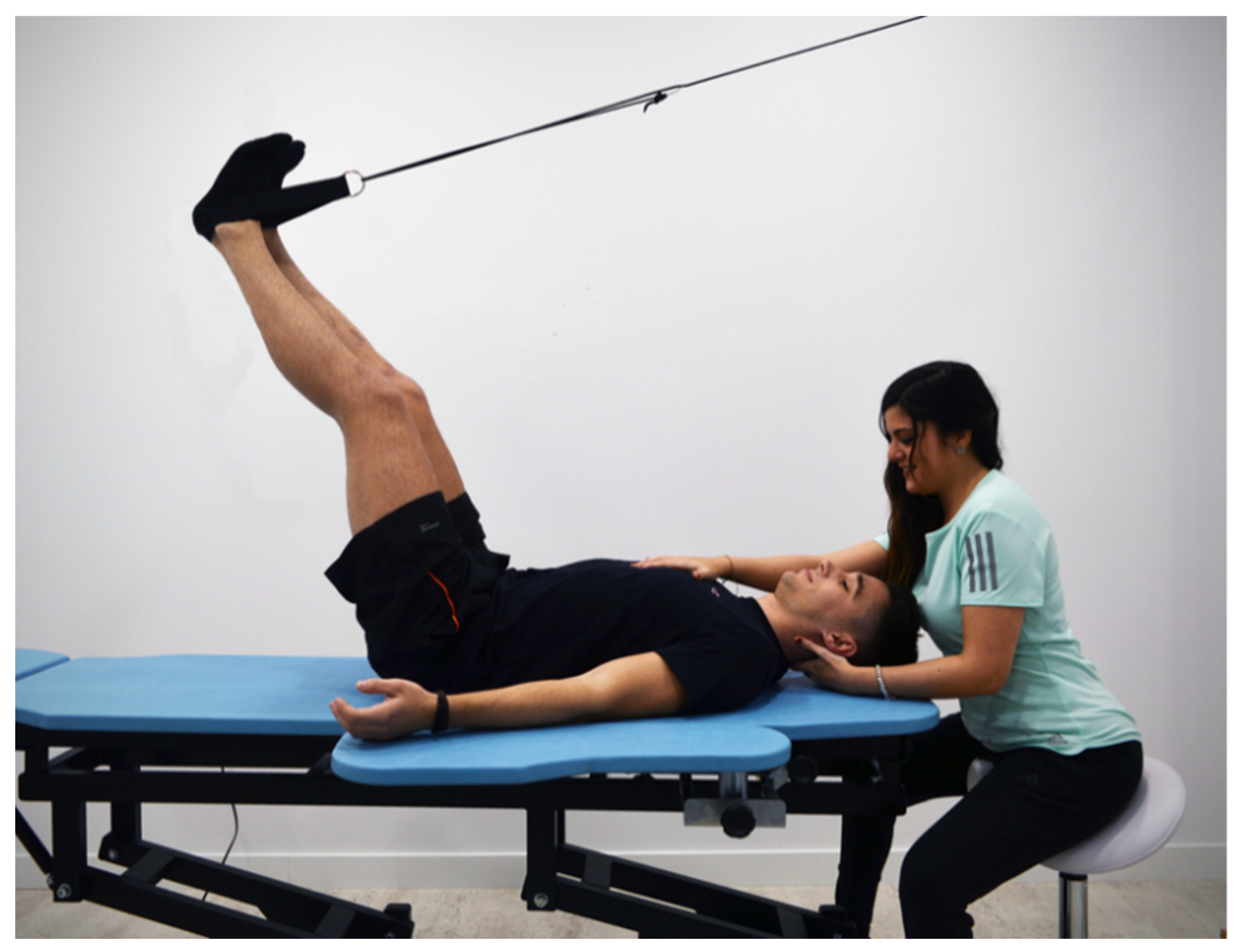
2.2. Therapeutic Management
2.3. Measurements
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Meglio, A.; Canavese, F. The immature spine: Growth and idiopathic scoliosis. Ann. Transl. Med. 2020, 8, 22. [Google Scholar] [CrossRef]
- Grossman, D.C.; Curry, S.J.; Owens, D.K.; Barry, M.J.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.J.; Kemper, A.R.; Krist, A.H.; Kurth, A.E.; et al. Screening for Adolescent Idiopathic Scoliosis: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 319, 165–172. [Google Scholar]
- Jagger, F.; Tsirikos, A.I.; Blacklock, S.; Urquhart, D.S. Adaptation to reduced lung function in children and young people with spinal deformity. J. Clin. Orthop. Trauma 2020, 11, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Newton, P.O.; Faro, F.D.; Gollogly, S.; Betz, R.R.; Lenke, L.G.; Lowe, T.G. Results of Preoperative Pulmonary Function Testing of Adolescents with Idiopathic Scoliosis. J. Bone Jt. Surg. 2005, 87, 1937–1946. [Google Scholar] [CrossRef]
- Weinstein, S.L.; Dolan, L.; Wright, J.G.; Dobbs, M.B. Effects of Bracing in Adolescents with Idiopathic Scoliosis. N. Engl. J. Med. 2013, 369, 1512–1521. [Google Scholar] [CrossRef] [Green Version]
- Lao, L.; Weng, X.; Qiu, G.; Shen, J. The role of preoperative pulmonary function tests in the surgical treatment of extremely severe scoliosis. J. Orthop. Surg. Res. 2013, 8, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrios, C.; Pérez-Encinas, C.; Maruenda, J.I.; Laguía, M. Significant Ventilatory Functional Restriction in Adolescents with Mild or Moderate Scoliosis during Maximal Exercise Tolerance Test. Spine 2005, 30, 1610–1615. [Google Scholar] [CrossRef]
- Kaelin, A.J. Adolescent idiopathic scoliosis: Indications for bracing and conservative treatments. Ann. Transl. Med. 2020, 8, 28. [Google Scholar] [CrossRef]
- Redding, G.J.; Mayer, O.H. Structure-Respiration Function Relationships Before and After Surgical Treatment of Early-onset Scoliosis. Clin. Orthop. Relat. Res. 2011, 469, 1330–1334. [Google Scholar] [CrossRef] [Green Version]
- Canavese, F. Idiopathic scoliosis. Ann. Transl. Med. 2020, 8, 21. [Google Scholar] [CrossRef]
- Ran, B.; Fan, Y.; Yuan, F.; Guo, K.; Zhu, X. Pulmonary function changes and its influencing factors after preoperative brace treatment in patients with adolescent idiopathic scoliosis: A retrospective case-control study. Medicine 2016, 95, e5088. [Google Scholar] [CrossRef]
- Day, J.M.; Fletcher, J.; Coghlan, M.; Ravine, T. Review of scoliosis-specific exercise methods used to correct adolescent idiopathic scoliosis. Arch. Physiother. 2019, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Dupuis, S.; Fortin, C.; Caouette, C.; LeClair, I.; Aubin, C. Global postural re-education in pediatric idiopathic scoliosis: A biomechanical modeling and analysis of curve reduction during active and assisted self-correction. BMC Musculoskelet. Disord. 2018, 19, 200. [Google Scholar] [CrossRef]
- Kim, K.-D.; Hwangbo, P.-N. Effects of the Schroth exercise on the Cobb’s angle and vital capacity of patients with idiopathic scoliosis that is an operative indication. J. Phys. Ther. Sci. 2016, 28, 923–926. [Google Scholar] [CrossRef] [Green Version]
- Moreno, M.A.; Catai, A.M.; Teodori, R.M.; Borges, B.L.A.; De Cesar, M.C.; Da Silva, E. Efeito de um programa de alongamento muscular pelo método de Reeducação Postural Global sobre a força muscular respiratória e a mobilidade toracoabdominal de homens jovens sedentários. J. Bras. Pneumol. 2007, 33, 679–686. [Google Scholar] [CrossRef] [Green Version]
- Teodori, R.M.; Moreno, M.A.; Fiore Junior, J.F.; Oliveira, A.C.S. Alongamento da musculatura inspiratoria por intermedio da reeducacao postural global (RPG). Braz. J. Phys. Ther. 2003, 7, 25–30. [Google Scholar]
- Durmuş, D.; Alaylı, G.; Uzun, O.; Tander, B.; Cantürk, F.; Bek, Y.; Erkan, L. Effects of two exercise interventions on pulmonary functions in the patients with ankylosing spondylitis. Jt. Bone Spine 2009, 76, 150–155. [Google Scholar] [CrossRef]
- Weniger, C.D.; Fujak, A.; Hofner, B.; Fuchs, M.; Forst, R.; Richter, R.H. Long-term Results of Conservative Therapy of Adolescent Idiopathic Scoliosis Using the Cheneau Brace. Klin. Padiatr. 2019, 231, 248–254. [Google Scholar] [CrossRef]
- King, H.A.; Moe, J.H.; Bradford, D.S.; Winter, R.B. The selection of fusion levels in thoracic idiopathic scoliosis. J. Bone Jt. Surg. Am. 1983, 65, 1302–1313. [Google Scholar] [CrossRef] [Green Version]
- Nici, L.; Donner, C.; Wouters, E.; ZuWallack, R.; Ambrosino, N.; Bourbeau, J.; Carone, M.; Celli, B.; Engelen, M.; Fahy, B.; et al. American Thoracic Society/European Respiratory Society Statement on Pulmonary Rehabilitation. Am. J. Respir. Crit. Care Med. 2006, 173, 1390–1413. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Yagci, G.; Demirkiran, G.; Yakut, Y. In-brace alterations of pulmonary functions in adolescents wearing a brace for idiopathic scoliosis. Prosthetics Orthot. Int. 2019, 43, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, B.M.; Araujo, G.S.; Sperandio, E.F.; Gotfryd, A.O.; Dourado, V.Z.; Vidotto, M. Impact of Scoliosis Severity on Functional Capacity in Patients with Adolescent Idiopathic Scoliosis. Pediatr. Exerc. Sci. 2018, 30, 243–250. [Google Scholar] [CrossRef]
- Souchard, P. Reeducación Postural Global, 1st ed.; Elsevier: Barcelona, Spain, 2012. [Google Scholar]
- Lieber, R.L.; Fridén, J. Muscle contracture and passive mechanics in cerebral palsy. J. Appl. Physiol. 2019, 126, 1492–1501. [Google Scholar] [CrossRef]
- Rassier, D.E. Sarcomere mechanics in striated muscles: From molecules to sarcomeres to cells. Am. J. Physiol. Physiol. 2017, 313, C134–C145. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hahn, D.; Power, G.A. Shortening-induced residual force depression in humans. J. Appl. Physiol. 2019, 126, 1066–1073. [Google Scholar] [CrossRef]
- Coksevim, N.H.; Durmus, D.; Kuru, O. Effects of global postural reeducation exercise and anti-TNF treatments on disease activity, function, fatigue, mobility, sleep quality and depression in patients with active Ankylosing spondylitis: A prospective follow-up study. J. Back Musculoskelet. Rehabil. 2018, 31, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Medina, G.; Perez-Cabezas, V.; Marin-Paz, A.-J.; Galán-Mercant, A.; Ruiz-Molinero, C.; Jimenez-Rejano, J.J. Effectiveness of Global Postural Reeducation in Ankylosing Spondylitis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2696. [Google Scholar] [CrossRef]
- Lomas-Vega, R.; Garrido-Jaut, M.V.; Rus, A.; Del-Pino-Casado, R. Effectiveness of Global Postural Re-education for Treatment of Spinal Disorders: A Meta-analysis. Am. J. Phys. Med. Rehabil. 2017, 96, 124–130. [Google Scholar] [CrossRef]



| N | Sex | Age (Year) | Height (cm) | Weight (Kg) | Body Mass Index (Kg/m2) | Main Curve Degrees | Type | Treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 12 | 1.48 | 42.0 | 19.17 | 17 | King IV | Brace |
| 2 | M | 14 | 1.71 | 52.0 | 17.78 | 16 | King IV | No intervention |
| 3 | M | 13 | 1.74 | 57.0 | 18.83 | 13 | King IV | No intervention |
| 4 | F | 14 | 1.51 | 81.0 | 35.52 | 12 | King IV | Brace |
| 5 | F | 14 | 1.65 | 62.0 | 22.77 | 16 | King I | GPR |
| 6 | F | 10 | 1.45 | 40.0 | 19.02 | 10 | King II | GPR |
| 7 | F | 14 | 1.67 | 44.0 | 15.78 | 27 | King I | Brace |
| 8 | F | 13 | 1.61 | 48.0 | 18.52 | 21 | King II | GPR |
| 9 | F | 12 | 1.44 | 42.0 | 20.25 | 18 | King I | No intervention |
| 10 | F | 13 | 1.54 | 44.0 | 18.55 | 10 | King II | No intervention |
| 11 | M | 14 | 1.67 | 45.5 | 16.31 | 10 | King IV | No intervention |
| 12 | M | 13 | 1.69 | 55.0 | 19.26 | 10 | King II | No intervention |
| 13 | F | 14 | 1.68 | 73.0 | 25.86 | 12 | King I | No intervention |
| 14 | F | 16 | 1.54 | 48.5 | 20.45 | 37 | King II | Brace |
| 15 | F | 16 | 1.65 | 64.0 | 23.50 | 16 | King II | GPR |
| Total | F = 73.3% M = 26.7% | 13 ± 12 (m ± SD) | 1.60 ± 0.10 (m ± SD) | 53.2 ± 12.2 (m ± SD) | 20.77 ± 4.86 (m ± SD) | 16 ± 7 (m ± SD) | I = 26.7% III = 40.0% IV = 33.3% | No intervention = 46.7% GPR = 26.7% Brace =26.7% |
| Age (Year) | Height (cm) | Weight (Kg) | Body Mass Index (Kg/m2) | Main Curve Degrees | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coef | p | Coef | p | Coef | p | Coef | p | Coef | p | |
| MIP | −0.131 | 0.643 | 0.113 | 0.689 | 0.229 | 0.412 | 0.318 | 0.248 | −0.266 | 0.338 |
| MIP ref | 0.290 | 0.294 | 0.650 | 0.009 ** | 0.727 | 0.002 ** | 0.215 | 0.442 | −0.293 | 0.289 |
| MIP (% pred) | −0.112 | 0.691 | 0.029 | 0.919 | 0.141 | 0.615 | 0.286 | 0.302 | −0.098 | 0.729 |
| FVC (L) | 0.224 | 0.422 | 0.673 | 0.006 ** | 0.485 | 0.067 | 0.007 | 0.980 | −0.400 | 0.140 |
| FVC % | −0.228 | 0.414 | 0.418 | 0.121 | −0.028 | 0.922 | −0.193 | 0.490 | −0.648 | 0.009 ** |
| FEV1 (L) | 0.207 | 0.459 | 0.426 | 0.113 | 0.465 | 0.081 | 0.229 | 0.413 | −0.185 | 0.510 |
| FEV1 % | −0.033 | 0.908 | 0.227 | 0.417 | 0.158 | 0.573 | 0.172 | 0.541 | −0.251 | 0.367 |
| FEV1/FVC | 0.345 | 0.208 | −0.004 | 0.990 | 0.347 | 0.205 | 0.393 | 0.147 | 0.338 | 0.217 |
| FEV1/FVC % | 0.252 | 0.364 | 0.065 | 0.819 | 0.342 | 0.212 | 0.365 | 0.181 | 0.249 | 0.370 |
| PEF (L/S) | 0.259 | 0.351 | 0.304 | 0.270 | 0.454 | 0.089 | 0.286 | 0.302 | −0.045 | 0.873 |
| PEF % | 0.207 | 0.460 | 0.135 | 0.633 | 0.412 | 0.127 | 0.404 | 0.135 | −0.053 | 0.850 |
| No Intervention (n = 7) | GPR (n = 4) | Brace (n = 4) | Ancova | Effect Size | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p-Value | Eta-2 | |
| MIP | 97.00 | 47.06 | 142.75 | 49.90 | 56.50 | 20.98 | 0.103 | 0.366 |
| MIP ref | 77.43 | 9.62 | 70.00 | 6.68 | 73.50 | 9.61 | 0.108 | 0.359 |
| MIP (% pred) | 126.11 | 57.30 | 201.12 | 55.22 | 78.46 | 33.10 | 0.045 * | 0.463 |
| FVC (L) | 3.57 | 0.78 | 2.17 | 1.22 | 2.77 | 0.37 | 0.146 | 0.319 |
| FVC % | 94.14 | 7.78 | 65.25 | 36.68 | 79.00 | 3.92 | 0.147 | 0.318 |
| FEV1 (L) | 2.82 | 0.85 | 1.94 | 1.21 | 1.98 | 0.87 | 0.510 | 0.126 |
| FEV1 % | 88.14 | 20.46 | 66.25 | 36.99 | 65.00 | 26.23 | 0.336 | 0.196 |
| FEV1/FVC | 79.05 | 14.97 | 89.16 | 12.18 | 69.57 | 27.24 | 0.216 | 0.264 |
| FEV1/FVC % | 93.29 | 17.78 | 102.75 | 13.57 | 80.75 | 30.72 | 0.239 | 0.249 |
| PEF L/S | 4.26 | 1.95 | 3.63 | 2.85 | 3.01 | 1.72 | 0.741 | 0.058 |
| PEF % | 66.43 | 29.06 | 61.00 | 42.43 | 50.00 | 31.18 | 0.589 | 0.101 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-Morillas, F.; de Oliveira-Sousa, S.L.; Andrade-Ortega, J.A.; Ibáñez-Vera, A.J.; Lomas-Vega, R.; Zagalaz-Anula, N. The Type of Conservative Management Could Be Related to the Strength of the Inspiratory Muscles of Adolescents with Idiopathic Scoliosis—A Case Series. Children 2021, 8, 1002. https://doi.org/10.3390/children8111002
León-Morillas F, de Oliveira-Sousa SL, Andrade-Ortega JA, Ibáñez-Vera AJ, Lomas-Vega R, Zagalaz-Anula N. The Type of Conservative Management Could Be Related to the Strength of the Inspiratory Muscles of Adolescents with Idiopathic Scoliosis—A Case Series. Children. 2021; 8(11):1002. https://doi.org/10.3390/children8111002
Chicago/Turabian StyleLeón-Morillas, Felipe, Silvana Loana de Oliveira-Sousa, Juan Alfonso Andrade-Ortega, Alfonso Javier Ibáñez-Vera, Rafael Lomas-Vega, and Noelia Zagalaz-Anula. 2021. "The Type of Conservative Management Could Be Related to the Strength of the Inspiratory Muscles of Adolescents with Idiopathic Scoliosis—A Case Series" Children 8, no. 11: 1002. https://doi.org/10.3390/children8111002








