Patterns of Response to Methylphenidate Administration in Children with ADHD: A Personalized Medicine Approach through Clustering Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Participants
2.1.2. Materials
- (1)
- Full-Scale Intelligence Quotient (FSIQ)
- (2)
- Clinical and behavioral measures
- (3)
- Neuropsychological measures
- (4)
- Stimulation protocol
- (5)
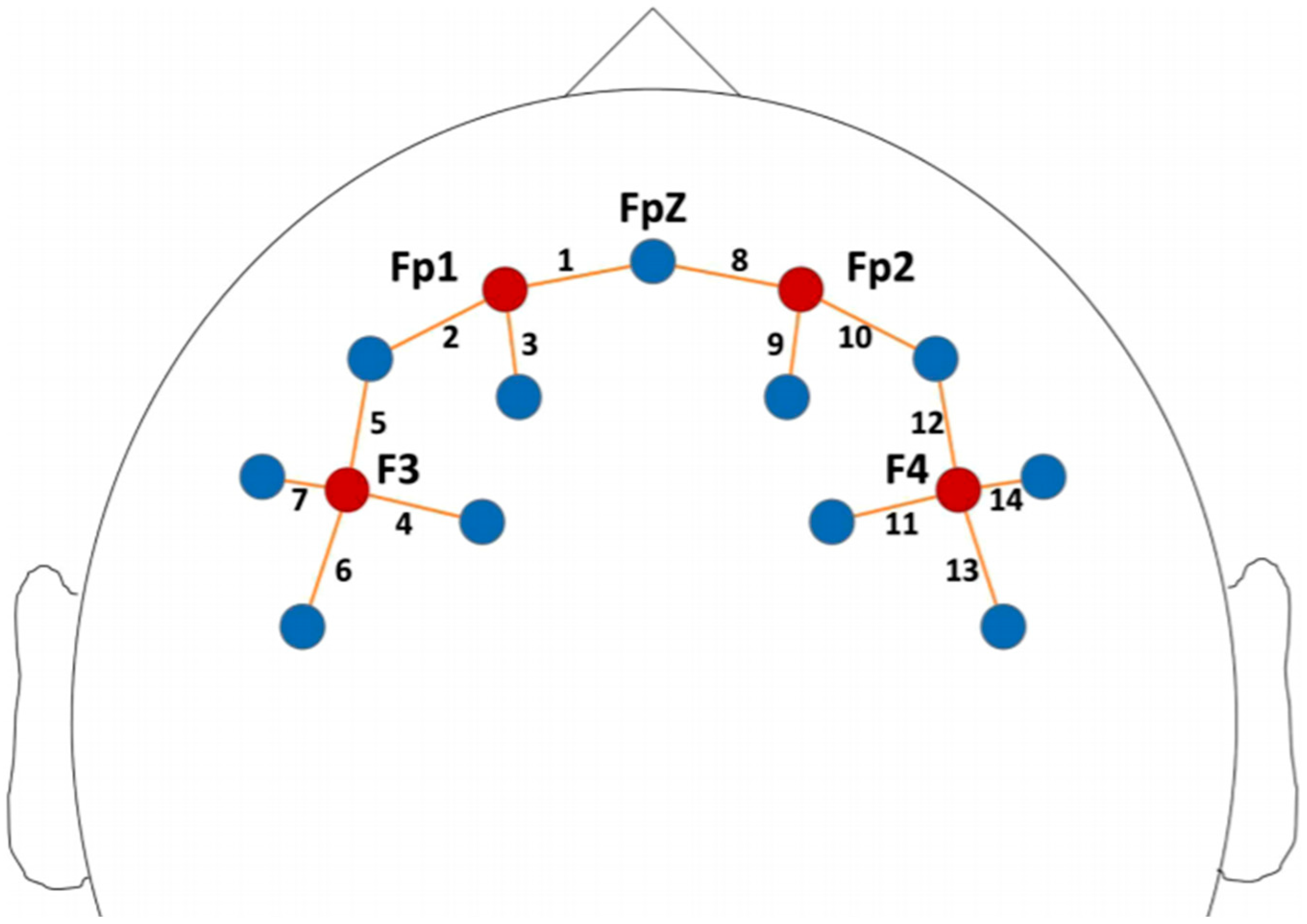
- fNIRS data acquisition
2.2. Statistical Analysis
3. Results
3.1. Between-Group Analyses
3.2. Within-Group Analyses
3.3. Clustering Analysis
3.3.1. Clinical Measures
3.3.2. Neuropsychological Measures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| CPRS-R Subscale | Model Solutions and Number of Clusters | BIC Value |
|---|---|---|
| Oppositional | Unequal variances, 3 | −353.021 |
| Unequal variances, 2 | −241.235 | |
| Equal variances, 3 | −241.235 | |
| Cognitive Problems–Inattention | Equal variances, 2 | −325.651 |
| Unequal variances, 2 | −328.59 | |
| Equal variances, 3 | −330.895 | |
| Hyperactivity–Impulsivity | Equal variances, 3 | −339.407 |
| Equal variances, 2 | −340.9 | |
| Unequal variances, 2 | −340.903 | |
| Perfectionism | Unequal variances, 2 | −307.189 |
| Equal variances, 2 | −309.316 | |
| Equal variances, 1 | −310.097 | |
| Social Problems | Unequal variances, 3 | −349.105 |
| Equal variances, 6 | −351.544 | |
| Equal variances, 8 | −352.87 | |
| Psychosomatic Problems | Unequal variances, 3 | −253.932 |
| Equal variances, 2 | −275.908 | |
| Equal variances, 8 | −277.611 | |
| ADHD Index | Equal variances, 2 | −323.539 |
| Equal variances, 3 | −324.537 | |
| Equal variances, 5 | −324.546 |
| Neuropsychological Scale | Model Solutions and Number of Clusters | BIC Value |
|---|---|---|
| NEPSY–Visual Attention | Equal variances, 6 | −184.236 |
| Equal variances, 5 | −188.422 | |
| Equal variances, 7 | −188.487 | |
| ANT–FA4L RT | Unequal variances, 2 | −461.331 |
| Equal variances, 3 | −463.189 | |
| Equal variances, 2 | −465.589 | |
| ANT–FA4L RT-SD | Equal variances, 5 | −376.117 |
| Equal variances, 6 | −377.499 | |
| Equal variances, 4 | −378.505 | |
| ANT–SAD RT | Equal variances, 7 | −172.838 |
| Unequal variances, 9 | −177.858 | |
| Unequal variances, 3 | −179.52 | |
| ANT–SAD RT-SD | Unequal variances, 1 | −121.226 |
| Equal variances, 1 | −121.226 | |
| Unequal variances, 9 | −121.613 |
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; DSM-5®; American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- Thapar, A.; Cooper, M. Attention deficit hyperactivity disorder. Lancet 2016, 387, 1240–1250. [Google Scholar] [CrossRef]
- Leopold, D.R.; Christopher, M.E.; Olson, R.K.; Petrill, S.A.; Willcutt, E.G. Invariance of ADHD symptoms across sex and age: A latent analysis of ADHD and impairment ratings from early childhood into adolescence. J. Abnorm. Child Psychol. 2018, 47, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Sayal, K.; Prasad, V.; Daley, D.; Ford, T.; Coghill, D. ADHD in children and young people: Prevalence, care pathways, and service provision. Lancet Psychiatry 2018, 5, 175–186. [Google Scholar] [CrossRef]
- Reale, L.; Bonati, M. ADHD prevalence estimates in Italian children and adolescents: A methodological issue. Ital. J. Pediatr. 2018, 44, 108. [Google Scholar] [CrossRef] [Green Version]
- American Academy of Pediatrics. ADHD: Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 2011, 128, 1007–1022. [Google Scholar] [CrossRef] [Green Version]
- Courtabessis, E.; Pupier, F.; Surig, L.; Picot, M.-C.; Nogué, E.; Macioce, V.; Stein, E.; Purper-Ouakil, D. Clinical factors associated with decision to recommend methylphenidate treatment for children with ADHD in France. Eur. Child Adolesc. Psychiatry 2017, 27, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Troncoso, E.; Guidi, M.; Alda-Díez, J.Á. Is psychological treatment efficacious for attention deficit hyperactivity disorder (ADHD)? Review of non-pharmacological treatments in children and adolescents with ADHD. Actas Esp. Psiquiatr. 2013, 41, 44–51. [Google Scholar] [PubMed]
- Faraone, S.V. The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci. Biobehav. Rev. 2018, 87, 255–270. [Google Scholar] [CrossRef]
- Arnsten, A.F.; Pliszka, S.R. Catecholamine influences on prefrontal cortical function: Relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol. Biochem. Behav. 2011, 99, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Schachter, H.M.; Pham, B.; King, J.; Langford, S.; Moher, D. How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis. Can. Med. Assoc. J. 2001, 165, 1475–1488. [Google Scholar]
- Barbaresi, W.J.; Katusic, S.K.; Colligan, R.C.; Weaver, A.L.; Leibson, C.L.; Jacobsen, S.J. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: Results from a population-based study. J. Dev. Behav. Pediatr. 2006, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Excellence NIfC. Attention Deficit Hyperactivity Disorder: The NICE Guideline on Diagnosis and Management of ADHD in Children, Young People and Adults; The British Psychological Society and the Royal College of Psychiatrists: London, UK, 2009. [Google Scholar]
- Storebø, O.J.; Krogh, H.B.; Ramstad, E.; Moreira-Maia, C.R.; Holmskov, M.; Skoog, M.; Nilausen, T.D.; Magnusson, F.L.; Zwi, M.; Gillies, D.; et al. Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta-analyses and trial sequential analyses of randomised clinical trials. BMJ 2015, 351, h5203. [Google Scholar] [CrossRef] [Green Version]
- Van Der Oord, S.; Prins, P.; Oosterlaan, J.; Emmelkamp, P. Efficacy of methylphenidate, psychosocial treatments and their combination in school-aged children with ADHD: A meta-analysis. Clin. Psychol. Rev. 2008, 28, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, Á.; Czobor, P.; Bálint, S.; Komlósi, S.; Simon, V.; Bitter, I. Pharmacotherapy of adult attention deficit hyperactivity disorder (ADHD): A meta-analysis. Int. J. Neuropsychopharmacol. 2009, 12, 1137–1147. [Google Scholar] [CrossRef] [Green Version]
- Hautmann, C.; Rothenberger, A.; Döpfner, M. An observational study of response heterogeneity in children with attention deficit hyperactivity disorder following treatment switch to modified-release methylphenidate. BMC Psychiatry 2013, 13, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii-Takahashi, A.; Takizawa, R.; Nishimura, Y.; Kawakubo, Y.; Hamada, K.; Okuhata, S.; Kawasaki, S.; Kuwabara, H.; Shimada, T.; Todokoro, A.; et al. Neuroimaging-aided prediction of the effect of methylphenidate in children with attention-deficit hyperactivity disorder: A randomized controlled trial. Neuropsychopharmacology 2015, 40, 2676–2685. [Google Scholar] [CrossRef] [Green Version]
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S.; Burgess, P.W. The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience. Ann. N. Y. Acad. Sci. 2020, 1464, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Grazioli, S.; Mauri, M.; Crippa, A.; Maggioni, E.; Molteni, M.; Brambilla, P.; Nobile, M. Light up ADHD: II. Neuropharmacological effects measured by near infrared spectroscopy: Is there a biomarker? J. Affect. Disord. 2019, 244, 100–106. [Google Scholar] [CrossRef]
- Monden, Y.; Dan, H.; Nagashima, M.; Dan, I.; Tsuzuki, D.; Kyutoku, Y.; Gunji, Y.; Yamagata, T.; Watanabe, E.; Momoi, M.Y. Right prefrontal activation as a neuro-functional biomarker for monitoring acute effects of methylphenidate in ADHD children: An fNIRS study. NeuroImage Clin. 2012, 1, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Nagashima, M.; Monden, Y.; Dan, I.; Dan, H.; Tsuzuki, D.; Mizutani, T.; Kyutoku, Y.; Gunji, Y.; Momoi, M.Y.; Watanabe, E.; et al. Neuropharmacological effect of methylphenidate on attention network in children with attention deficit hyperactivity disorder during oddball paradigms as assessed using functional near-infrared spectroscopy. Neurophotonics 2014, 1, 015001. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, M.D.; Scholkmann, F.; Labruyère, R. Signal Processing in Functional Near-Infrared Spectroscopy (fNIRS): Methodological differences lead to different statistical results. Front. Hum. Neurosci. 2018, 11, 641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walkup, J.T.; Stossel, L.; Rendleman, R. Beyond rising rates: Personalized medicine and public health approaches to the diagnosis and treatment of attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 14–16. [Google Scholar] [CrossRef]
- Keizer, A.W. Standardization and personalized medicine using quantitative EEG in clinical settings. Clin. EEG Neurosci. 2021, 52, 82–89. [Google Scholar] [CrossRef]
- Myer, N.M.; Boland, J.R.; Faraone, S.V. Pharmacogenetics predictors of methylphenidate efficacy in childhood ADHD. Mol. Psychiatry 2017, 23, 1929–1936. [Google Scholar] [CrossRef]
- Wong, H.K.; Tiffin, P.A.; Chappell, M.J.; Nichols, T.E.; Welsh, P.R.; Doyle, O.M.; Lopez-Kolkovska, B.C.; Inglis, S.K.; Coghill, D.; Shen, Y.; et al. Personalized medication response prediction for attention-deficit hyperactivity disorder: Learning in the model space vs. learning in the data space. Front. Physiol. 2017, 8, 199–220. [Google Scholar] [CrossRef] [Green Version]
- Chekroud, A.M.; Bondar, J.; Delgadillo, J.; Doherty, G.; Wasil, A.; Fokkema, M.; Cohen, Z.; Belgrave, D.; DeRubeis, R.; Iniesta, R.; et al. The promise of machine learning in predicting treatment outcomes in psychiatry. World Psychiatry 2021, 20, 154–170. [Google Scholar] [CrossRef]
- Berry, M.W.; Mohamed, A.; Yap, B.W. Supervised and Unsupervised Learning for Data Science; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Jain, A.K.; Dubes, R.C. Algorithms for Clustering Data; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 1998. [Google Scholar]
- Jain, A.K.; Murty, M.N.; Flynn, P.J. Data clustering: A review. ACM Comput. Surv. 1999, 31, 264–323. [Google Scholar] [CrossRef]
- Shirkhorshidi, A.S.; Aghabozorgi, S.; Wah, T.Y. A comparison study on similarity and dissimilarity measures in clustering continuous data. PLoS ONE 2015, 10, e0144059. [Google Scholar] [CrossRef] [Green Version]
- Reimherr, F.W.; Marchant, B.K.; Gift, T.E.; Steans, T.A.; Wender, P.H. Types of adult attention-deficit hyperactivity disorder (ADHD): Baseline characteristics, initial response, and long-term response to treatment with methylphenidate. ADHD Atten. Deficit Hyperact. Disord. 2015, 7, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Sharma, V.; Ryan, N.D. Predicting methylphenidate response in ADHD using machine learning approaches. Int. J. Neuropsychopharmacol. 2015, 18, pyv052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melnykov, V.; Maitra, R. Finite mixture models and model-based clustering. Stat. Surv. 2010, 4, 80–116. [Google Scholar] [CrossRef]
- Mauri, M.; Grazioli, S.; Crippa, A.; Bacchetta, A.; Pozzoli, U.; Bertella, S.; Gatti, E.; Maggioni, E.; Rosi, E.; Diwadkar, V.; et al. Hemodynamic and behavioral peculiarities in response to emotional stimuli in children with attention deficit hyperactivity disorder: An fNIRS study. J. Affect. Disord. 2020, 277, 671–680. [Google Scholar] [CrossRef]
- Busner, J.; Targum, S.D. The clinical global impressions scale: Applying a research tool in clinical practice. Psychiatry 2007, 4, 28–37. [Google Scholar]
- Goodman, R.; Ford, T.; Richards, H.; Gatward, R.; Meltzer, H. The development and wellbeing assessment: Description and initial validation of an integrated assessment of child and adolescent psychopathology. J. Child Psychol. Psychiatry 2000, 41, 645–655. [Google Scholar] [CrossRef]
- Hollingshead, A.B. Four Factor Index of Social Status; Yale University: New Haven, CT, USA, 1975. [Google Scholar]
- Wechsler, D. Wechsler Intelligence Scale for Children–IV (WISC-IV), 4th ed.; Italian Edition; Organizzazioni Speciali: Florence, Italy, 2012. [Google Scholar]
- Wechsler, D. Wechsler Intelligence Scale for Children–III (WISC-III), 3th ed.; Italian Edition; Organizzazioni Speciali: Florence, Italy, 2006. [Google Scholar]
- Crawford, J.R.; Anderson, V.; Rankin, P.M.; Macdonald, J. An index-based short-form of the WISC-IV with accompanying analysis of the reliability and abnormality of differences. Br. J. Clin. Psychol. 2010, 49, 235–258. [Google Scholar] [CrossRef]
- Conners, C.K. Conners’ Rating Scales—Revised; American Psychological Association (APA): Washington, DC, USA, 2012. [Google Scholar]
- Nobile, M.; Alberti, B.; Zuddas, A. CRS-R. Conners’ Rating Scales. Revised. Manuale; Giunti Editore: Florence, Italy, 2007. [Google Scholar]
- Endicott, J.; Spitzer, R.L.; Fleiss, J.L.; Cohen, J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch. Gen. Psychiatry 1976, 33, 766–771. [Google Scholar] [CrossRef]
- De Sonneville, L.M.J. ANT 2.1—Amsterdam Neuropsychological Tasks; Sonar: Amstelveen, The Netherlands, 2000. [Google Scholar]
- Crippa, A.; Agostoni, C.; Mauri, M.; Molteni, M.; Nobile, M. Polyunsaturated fatty acids are associated with behavior but not with cognition in children with and without ADHD: An Italian study. J. Atten. Disord. 2018, 22, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Crippa, A.; Tesei, A.; Sangiorgio, F.; Salandi, A.; Trabattoni, S.; Grazioli, S.; Agostoni, C.; Molteni, M.; Nobile, M. Behavioral and cognitive effects of docosahexaenoic acid in drug-naïve children with attention-deficit/hyperactivity disorder: A randomized, placebo-controlled clinical trial. Eur. Child Adolesc. Psychiatry 2018, 28, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Korkman, M.; Kirk, U.; Kemp, S. NEPSY-II: Administration Manual; Harcourt Assessment: San Antonio, TX, USA, 2007. [Google Scholar]
- Soloff, P.H.; White, R.; Omari, A.; Ramaseshan, K.; Diwadkar, V.A. Affective context interferes with brain responses during cognitive processing in borderline personality disorder: fMRI evidence. Psychiatry Res. Neuroimaging 2015, 233, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Huppert, T.J.; Diamond, S.; Franceschini, M.A.; Boas, D.A. HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009, 48, D280–D298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piazza, C.; Bacchetta, A.; Crippa, A.; Mauri, M.; Grazioli, S.; Reni, G.; Nobile, M.; Bianchi, A.M. Preprocessing Pipeline for fNIRS Data in Children. In Proceedings of the VI Latin American Congress on Biomedical Engineering CLAIB 2014, Paraná, Argentina, 29–31 October 2014; pp. 235–244. [Google Scholar] [CrossRef]
- Mauri, M.; Nobile, M.; Bellina, M.; Crippa, A.; Brambilla, P. Light up ADHD: I. Cortical hemodynamic responses measured by functional Near Infrared Spectroscopy (fNIRS): Special section on “Translational and Neuroscience Studies in Affective Disorders”. J. Affect. Disord. 2018, 234, 358–364. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: http://www.R-project.org (accessed on 30 August 2021).
- Scrucca, L.; Fop, M.; Murphy, T.B.; Raftery, A.E. mclust 5: Clustering, classification and density estimation using Gaussian finite mixture models. R J. 2016, 8, 289–317. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, M.; Jain, A.K. Unsupervised learning of finite mixture models. IEEE Trans. Pattern Anal. Mach. Intell. 2002, 24, 381–396. [Google Scholar] [CrossRef] [Green Version]
- Clatworthy, J.; Buick, D.; Hankins, M.; Weinman, J.A.; Horne, R. The use and reporting of cluster analysis in health psychology: A review. Br. J. Heal. Psychol. 2005, 10, 329–358. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, B.; Abikoff, H.B.; Chuang, S.Z.; Kollins, S.H.; McCracken, J.T.; Riddle, M.A.; Greenhill, L.L. Effectiveness of methylphenidate in the 10-month continuation phase of the Preschoolers with Attention-Deficit/Hyperactivity Disorder Treatment Study (PATS). J. Child. Adolesc. Psychopharmacol. 2007, 17, 593–604. [Google Scholar] [CrossRef]
- Ghuman, J.K.; Aman, M.G.; Lecavalier, L.; Riddle, M.A.; Gelenberg, A.; Wright, R.; Rice, S.; Ghuman, H.S.; Fort, C. Randomized, placebo-controlled, crossover study of methylphenidate for attention-deficit/hyperactivity disorder symptoms in preschoolers with developmental disorders. J. Child Adolesc. Psychopharmacol. 2009, 19, 329–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efron, D.; Jarman, F.C.; Barker, M.J. Medium-term outcomes are comparable with short-term outcomes in children with attention deficit hyperactivity disorder treated with stimulant medication. J. Paediatr. Child Health 2000, 36, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.; Baird, G.; Rankin, P.M.; Isaacs, E. Immediate effects of methylphenidate on cognitive attention skills of children with attention-deficit–hyperactivity disorder. Dev. Med. Child Neurol. 1999, 47, 408–414. [Google Scholar] [CrossRef]
- Di Martino, A.; Ghaffari, M.; Curchack, J.; Reiss, P.; Hyde, C.; Vannucci, M. Decomposing intra-subject variability in children with attention-deficit/hyperactivity disorder. Biol. Psychiatry 2008, 64, 607–614. [Google Scholar] [CrossRef] [Green Version]
- Pievsky, M.A.; McGrath, R.E. The neurocognitive profile of attention-deficit/hyperactivity disorder: A review of meta-analyses. Arch. Clin. Neuropsychol. 2018, 33, 143–157. [Google Scholar] [CrossRef] [Green Version]
- Mueller, S.; Costa, A.; Keeser, D.; Pogarell, O.; Berman, A.; Coates, U.; Reiser, M.F.; Riedel, M.; Möller, H.-J.; Ettinger, U.; et al. The effects of methylphenidate on whole brain intrinsic functional connectivity. Hum. Brain Mapp. 2014, 35, 5379–5388. [Google Scholar] [CrossRef]
- Finke, K.; Dodds, C.M.; Bublak, P.; Regenthal, R.; Baumann, F.; Manly, T.; Müller, U. Effects of modafinil and methylphenidate on visual attention capacity: A TVA-based study. Psychopharmacology 2010, 210, 317–329. [Google Scholar] [CrossRef]
- Friedman, L.M.; McBurnett, K.; Dvorsky, M.R.; Hinshaw, S.P.; Pfiffner, L.J. Learning Disorder Confers Setting-Specific Treatment Resistance for Children with ADHD, Predominantly Inattentive Presentation. J. Clin. Child Adolesc. Psychol. 2020, 49, 854–867. [Google Scholar] [CrossRef]
- Childress, A.C.; Komolova, M.; Sallee, F.R. An update on the pharmacokinetic considerations in the treatment of ADHD with long-acting methylphenidate and amphetamine formulations. Expert Opin. Drug Metab. Toxicol. 2019, 15, 937–974. [Google Scholar] [CrossRef] [Green Version]
| T0 | T1 | ||
|---|---|---|---|
| ADHD Group | TD Group | ADHD Group | |
| FSIQ | ✓ | ✓ | X |
| SES | ✓ | ✓ | X |
| Clinical | ✓ | ✓ | ✓ |
| CPRS-R | ✓ | ✓ | ✓ |
| Neuropsychological | ✓ | ✓ | ✓ |
| fNIRS | ✓ | ✓ | ✓ |
| T0 | T1 | Statistic Value | p | |
|---|---|---|---|---|
| C-GAS Value (percentage of subjects) | 4 (18%) 5 (76%) 6 (6%) | 5 (13%) 6 (13%) 7 (25%) 8 (30%) 9 (19%) | 0 a | <0.001 |
| CGI-S Value (percentage of subjects) | 4 (18%) 5 (59%) 6 (23%) | 2 (6%) 3 (19%) 4 (56%) 5 (19%) | 136 a | <0.001 |
| CPRS-R (mean ± SD) | ||||
| Oppositional | 72.88 ± 16.55 | 61.52 ± 17.11 | 158 a | 0.002 |
| Cognitive problems | 81.08 ± 11.62 | 70.57 ± 15.22 | 201.5 a | <0.001 |
| Hyperactivity–Impulsivity | 77.33 ± 11.50 | 68.48 ± 17.06 | 161 a | 0.008 |
| Anxious–Shy | 52.25 ± 11.10 | 48.05 ± 10.05 | 98.5 a | 0.31 |
| Perfectionism | 53.66 ± 11.71 | 45.47 ± 8.28 | 111 a | 0.004 |
| Social Problems | 71.29 ± 19.89 | 62.90 ± 18.96 | 99 a | 0.028 |
| Psychosomatic Problems | 53.04 ± 13.80 | 47.00 ± 6.20 | 76.5 a | 0.032 |
| ADHD index | 81.54 ± 9.61 | 71.52 ± 13.70 | 201 a | <0.001 |
| NEPSY (mean ± SD) | ||||
| Visual Attention | 9.30 ± 3.52 | 11.95 ± 3.57 | 5.0 a | <0.001 |
| ANT (ms) (mean ± SD): Baseline Speed | ||||
| RT | 406.35 ± 134.82 | 384 ± 130.34 | 73.0 a | 0.890 |
| SD of RT | 234.30 ± 195.08 | 192.52 ± 160.83 | 75.0 a | 0.963 |
| ANT (ms) (mean ± SD): Focused Attention Four Letters | ||||
| RT correct responses | 1304.89 ± 491.08 | 1001.77 ± 318.87 | 147.0 a | <0.001 |
| SD of correct responses RT | 558 ± 304.17 | 341.66 ± 173.54 | 146.5 a | <0.001 |
| ANT (ms) (mean ± SD): Shifting Attentional Set—Visual | ||||
| RT inhibition | 270.81 ± 292.63 | 258. 53 ± 237.83 | 62.0 a | 0.583 |
| RT flexibility | 453.06 ± 492.93 | 457.43 ± 249.18 | 63.5 a | 0.221 |
| ANT (ms) (mean ± SD): Sustained Attention Dots | ||||
| Time × Series | 17.27 ± 5.96 | 14.65 ± 5.91 | 119.0 a | 0.009 |
| SD | 3.88 ± 1.45 | 3.01 ± 1.35 | 108.5 a | 0.006 |
| Neurophysiological characteristics | ||||
| fNIRS signal (mean ± SD) | ||||
| Right prefrontal | 1.43 ± 3.64 | 0.31 ± 2.72 | 64.0 a | 0.850 |
| Right frontal | 0.78 ± 3.70 | 0.31 ± 2.72 | 60.0 a | 0.670 |
| Left prefrontal | 0.95 ± 3.58 | −0.88 ± 3.90 | 91.0 a | 0.252 |
| Left frontal | 1.08 ± 2.44 | −0.49 ± 3.41 | 62.0 a | 0.273 |
| Cognitive problems | Cluster | 1 | 2 |
| subjects | 58% | 42% | |
| Mean before MPH (± sd) | 72.99 (±6.79) | 91.93 (±6.79) | |
| Mean after MPH (± sd) | 59.54 (±6.79) | 85.95 (±6.79) | |
| Comorbidities | SLD: 25% * | SLD: 80% * | |
| Perfectionism | Cluster | 1 | 2 |
| subjects | 57% | 43% | |
| Mean before MPH (± sd) | 43.09 (±8) | 76.15 (±3.38) | |
| Mean after MPH (± sd) | 40.41 (±8) | 45.75 (±3.38) | |
| ADHD index | Cluster | 1 | 2 |
| subjects | 51% | 49% | |
| Mean before MPH (± sd) | 87.03 (±6.81) | 74.34 (±6.81) | |
| Mean after MPH (± sd) | 82.85 (±6.81) | 59.76 (±6.81) |
| ANT–FA4L RT | Cluster | 1 | 2 |
| subjects | 63% | 37% | |
| Mean before MPH (± sd) | 1586.08 (±252) | 804.52 (±94) | |
| Mean after MPH (± sd) | 1231.25 (±252) | 640.67 (±94) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grazioli, S.; Rosi, E.; Mauri, M.; Crippa, A.; Tizzoni, F.; Tarabelloni, A.; Villa, F.M.; Chiapasco, F.; Reimers, M.; Gatti, E.; et al. Patterns of Response to Methylphenidate Administration in Children with ADHD: A Personalized Medicine Approach through Clustering Analysis. Children 2021, 8, 1008. https://doi.org/10.3390/children8111008
Grazioli S, Rosi E, Mauri M, Crippa A, Tizzoni F, Tarabelloni A, Villa FM, Chiapasco F, Reimers M, Gatti E, et al. Patterns of Response to Methylphenidate Administration in Children with ADHD: A Personalized Medicine Approach through Clustering Analysis. Children. 2021; 8(11):1008. https://doi.org/10.3390/children8111008
Chicago/Turabian StyleGrazioli, Silvia, Eleonora Rosi, Maddalena Mauri, Alessandro Crippa, Federica Tizzoni, Arianna Tarabelloni, Filippo Maria Villa, Federica Chiapasco, Maria Reimers, Erika Gatti, and et al. 2021. "Patterns of Response to Methylphenidate Administration in Children with ADHD: A Personalized Medicine Approach through Clustering Analysis" Children 8, no. 11: 1008. https://doi.org/10.3390/children8111008
APA StyleGrazioli, S., Rosi, E., Mauri, M., Crippa, A., Tizzoni, F., Tarabelloni, A., Villa, F. M., Chiapasco, F., Reimers, M., Gatti, E., Bertella, S., Molteni, M., & Nobile, M. (2021). Patterns of Response to Methylphenidate Administration in Children with ADHD: A Personalized Medicine Approach through Clustering Analysis. Children, 8(11), 1008. https://doi.org/10.3390/children8111008






