Biomarkers and Fever in Children with Cancer: Kinetics and Levels According to Final Diagnosis
Abstract
:1. Background
2. Materials and Methods
2.1. Study Design and Patients
2.2. Diagnostic Groups
2.3. Definitions
2.4. Interpretation of Biomarker Values
2.5. Laboratory Methods
2.6. Statistical Analysis
3. Results
3.1. Descriptive Study: Epidemiological Data
3.2. Biomarkers and Final Diagnosis
4. Discussion
4.1. Biomarker Levels and Kinetics
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basic Figures of Childhood Cancer. Childhood Cancer in Spain: Questions and Data. National Registry of Childhood Tumours-SEHOP. Available online: https://www.uv.es/rnti/pdfs/B1.05 (accessed on 12 May 2021).
- Spanish Registry of Childhood Tumors RETI-SEHOP. Available online: https://www.uv.es/rnti/cifrasCancer.html (accessed on 12 May 2021).
- Clyne, B.; Olshaker, J.S. The C-reactive protein. J. Emerg. Med. 1999, 17, 1019–1025. [Google Scholar] [CrossRef]
- Manian, F.A. A prospective study of daily measurement of c-reactive protein in serum of adults with neutropenia. Clin. Infect. Dis. 1995, 21, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Chawes, B.L.; Rechnitzer, C.; Schmiegelow, K.; Tvede, M. Procalcitonin tiltidligdiagnostikafbakteriæmi hos børn med cancer. Ugeskr. Laeger. 2007, 169, 138–142. [Google Scholar] [PubMed]
- Vyles, D.; Gnagi, F.; Bulloch, B.; Muenzer, J.; Hu, C. Procalcitonin as a marker of bacteremia in patients with fever and acute lymphoblastic leukemia. Ped. Emerg. Care 2016, 32, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Tan, T.T.; Lim, S.T.; Farid, M.; Tao, M.; Quek, R.; Tang, T. Role of procalcitonin in differentiating between infectious and noninfectious fevers among patients with lymphoma. Pharmacotherapy 2017, 37, 908–915. [Google Scholar] [CrossRef]
- Daef, E.A.; Elsherbiny, N.M.; Agban, M.N.; Riad, K.F.; Mohammed, L.F. Bloodstream infections in febrile neutropenic pediatric cancer patients: Microbiological and sepsis biomarkers insight. Egypt J. Immunol. 2018, 25, 21–34. [Google Scholar]
- Hinson, J.P.; Kapas, S.; Smith, D.M. Adrenomedullin, a multifunctional regulatory peptide. Endocrinol. Rev. 2000, 21, 138–167. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Morgenthaler, N.G.; Struck, J.; Harbarth, S.; Bergmann, A.; Müller, B. Mid-regional pro-adrenomedullin as a prognostic marker in sepsis: An observational study. Crit. Care 2005, 9, R816. [Google Scholar] [CrossRef] [Green Version]
- Guignant, C.; Voirin, N.; Venet, F.; Poitevin, F.; Malcus, C.; Bohé, J.; Monneret, G. Assessment of pro-vasopressin and pro-adrenomedullin as predictors of 28-day mortality in septic shock patients. Crit. Care Med. 2009, 35, 1859–1867. [Google Scholar] [CrossRef]
- Chaftari, A.M.; Hachem, R.; Reitzel, R.; Jordan, M.; Jiang, Y.; Yousif, A.; Raad, I. Role of procalcitonin and interleukin-6 in predicting cancer, and its progression independent of infection. PLoS ONE 2015, 10, e0130999. [Google Scholar] [CrossRef]
- Goldstein, B.; Giroir, B.; Randolph, A. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med. 2005, 6, 2–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohli, V.; Singhi, S.; Sharma, P.; Ganguly, N.K. Value of serum C-reactive protein concentrations in febrile children without apparent focus. Ann. Trop. Paediatr. 1993, 13, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Leli, C.; Ferranti, M.; Marrano, U.; Al Dhahab, Z.S.; Bozza, S.; Cenci, E.; Mencacci, A. Diagnostic accuracy of presepsin (sCD14-ST) and procalcitonin for prediction of bacteraemia and bacterial DNAaemia in patients with suspected sepsis. J. Med. Microbiol. 2016, 65, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Harwood, P.J.; Loughenbury, P.; van Griensven, M.; Krettek, C.; Pape, H.C. Correlation between IL-6 levels and the systemic inflammatory response score: Can an IL-6 cutoff predict a SIRS state? J. Trauma 2008, 65, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Travaglino, F.; de Berardinis, B.; Magrini, L.; Bongiovanni, C.; Candelli, M.; Silveri, N.G.; Di Somma, S. Utility of procalcitonin (PCT) and Mid regional pro-Adrenomedullin (MR-proADM) in risk stratification of critically ill febrile patients in Emergency Department (ED). A comparison with APACHE II score. BMC Infect. Dis. 2012, 12, 184. [Google Scholar] [CrossRef] [Green Version]
- Sariego-Jamardo, A.; Rey, C.; Medina, A.; Mayordomo-Colunga, J.; Concha-Torre, A.; Prieto, B.; Vivanco-Allende, A. C-reactive protein, procalcitonin and interleukin-6 kinetics in pediatric postoperative patients. J. Crit. Care 2017, 41, 119–123. [Google Scholar] [CrossRef]
- Aznar-Oroval, E.; Sánchez-Yepes, M.; Lorente-Alegre, P.; San Juan-Gadea, M.C.; Ortiz-Muñoz, B.; Prez-Ballestero, P.; Maíquez-Richart, J. Valor diagnóstico de la procalcitonina, la interleucina 8, la interleucina 6 y la proteína C reactivaen la deteccin de bacteriemia y fungemia enpacientes con cáncer. EnfInfec. Microbiol. Clin. 2010, 28, 273–277. [Google Scholar] [CrossRef]
- Chirouze, C.; Schuhmacher, H.; Rabaud, C.; Gil, H.; Khayat, N.; Estavoyer, J.M.; Hoen, B. Low serum procalcitonin level accurately predicts the absence of bacteremia in adult patients with acute fever. Clin. Infect. Dis. 2002, 35, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Gerique, J.A.G.; Espejo, M.O.; Rodríguez, M.I.T.; Álvarez, J.G.; Castellanos-Ortega, Á.; Suberviola, B.; Teja, J.L. Evaluación de la capacidaddiagnóstica y pronóstica de procalcitonina, proteína C reactiva, interleucina-6 y proteínaligadora del lipopolisacáridoenpacientes con sospecha de sepsis. Lab. Clin. 2010, 3, 12–19. [Google Scholar] [CrossRef]
- von Lilienfeld-Toal, M.; Dietrich, M.P.; Glasmacher, A.; Lehmann, L.; Breig, P.; Hahn, C.; Stuber, F. Markers of bacteremia in febrile neutropenic patients with hematological malignancies: Procalcitonin and IL-6 are more reliable than C-reactive protein. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 539–544. [Google Scholar] [CrossRef]
- Cost, C.R.; Stegner, M.M.; Leonard, D.; Leavey, P. IL-8 predicts pediatric oncology patients with febrile neutropenia at low risk for bacteremia. J. Ped. Hematol. Oncol. 2013, 35, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.W.; Wu, J.Y.; Chen, C.K.; Huang, S.L.; Hsu, S.C.; Lee, M.T.G.; Lee, C.C. Does procalcitonin, C-reactive protein, or interleukin-6 test have a role in the diagnosis of severe infection in patients with febrile neutropenia? A systematic review and meta-analysis. Support Care Cancer 2015, 23, 2863–2872. [Google Scholar] [CrossRef]
- Altman, D.G.; Riley, R.D. Primer: An evidence-based approach to prognostic markers. Nat. Rev. Clin. Oncol. 2005, 2, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Araujo, O.R.D.; Salomaõ, R.; Brunialti, M.K.C.; Silva, D.C.B.D.; Senerchia, A.A.; Moraes Costa Carlesse, F.A.D.; Petrilli, A.S. Cytokine kinetics in febrile neutropenic children: Insights on the usefulness as sepsis biomarkers, influence of filgrastim, and behavior of the IL-23/IL-17 pathway. Mediat. Inflamm. 2017, 2017, 8291316. [Google Scholar] [CrossRef] [PubMed]
- Al Shuaibi, M.; Bahu, R.R.; Chaftari, A.M.; Al Wohoush, I.; Shomali, W.; Jiang, Y.; Raad, I. Pro-adrenomedullin as a novel biomarker for predicting infections and response to antimicrobials in febrile patients with hematologic malignancies. Clin. Infect. Dis. 2013, 56, 943–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debiane, L.; Hachem, R.Y.; Al Wohoush, I.; Shomali, W.; Bahu, R.R.; Jiang, Y.; Raad, I. The utility of proadrenomedullin and procalcitonin in comparison to C-reactive protein as predictors of sepsis and bloodstream infections in critically ill patients with cancer. Crit. Care Med. 2014, 42, 2500–2507. [Google Scholar] [CrossRef] [PubMed]
- Rey, C.; García-Hernández, I.; Concha, A.; Martínez-Camblor, P.; Botrán, M.; Medina, A.; López-Herce, J. Pro-adrenomedullin, pro-endothelin-1, procalcitonin, C-reactive protein and mortality risk in critically ill children: A prospective study. Crit. Care 2013, 17, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Reinhart, K.; Bauer, M.; Riedemann, N.C.; Hartog, C.S. New approaches to sepsis: Molecular diagnostics and biomarkers. Clin. Microbiol. Rev. 2012, 25, 609–634. [Google Scholar] [CrossRef] [Green Version]
| PATIENTS (n = 37) | |
|---|---|
| Male, n (%) | 14 (37.8%) |
| Mean (95% CI)/Median (IQR) in years at the first febril episode | 8 (6.4–9.5)/7.3 (8.5) |
| Solid tumor/HL | 19 (51.3%) |
| ALL/AML/NHL | 18 (48.6%) |
| EPISODES (n = 134) | |
| Solid tumor/HL | 74 (55.2%) |
| ALL/AML/NHL | 60 (44.7%) |
| Comorbidities, n (%) | 86 (64.1%) |
| No control disease, n (%) | 23 (17.1%) |
| Phase of treatment: | |
| ALL/AML/NHL induction, n (%) | 19 (14.1%) |
| Maintenance leukaemia, | 26 (19.4%) |
| BMT, n (%) | 15 (11.1%) |
| Solid tumour/HL, n (%) | 74 (55.2%) |
| Symptoms | 81 (60.4%) |
| Yes, n (%) | |
| Number of hours previous assistance | |
| Mean (95% CI)/Median (IQR) | 4.4 (3–5.7)/2 (4) |
| Transfusion (blood +/−platelets) | |
| Yes, n (%) | 43 (32.1%) |
| GCS-F | |
| Yes, (%) | 28 (20.9%) |
| Final diagnoses: | 38 (28.3%) |
| Bacterial infection, n (%) | 8 (5.9%) |
| SIRS, n (%) | 88 (65.6%) |
| Non bacterial infection, n (%) |
| BACTERIAL INFECTION (n = 38) | NO BACTERIAL INFECTION (n = 88) | SIRS (n = 8) | ||
|---|---|---|---|---|
| Bacteriemia | 6 (4.4%) | Cutaneous infection | 2 (1.4%) | 8 (5.9%) |
| Catheter infection | 3 (2.2%) | Fever of unknown origin | 36 (26.8%) | |
| Gastroenteritis | 4 (2.9%) | Oropharyngeal candidiasis | 2 (1.4%) | |
| Pneumonia | 1 (0.7%) | Upper respiratory infection | 48 (35.8%) | |
| Throat infection | 2 (1.4%) | |||
| Urinary tract infection | 18 (13.4) | |||
| Sepsis | 4 (2.9%) | |||
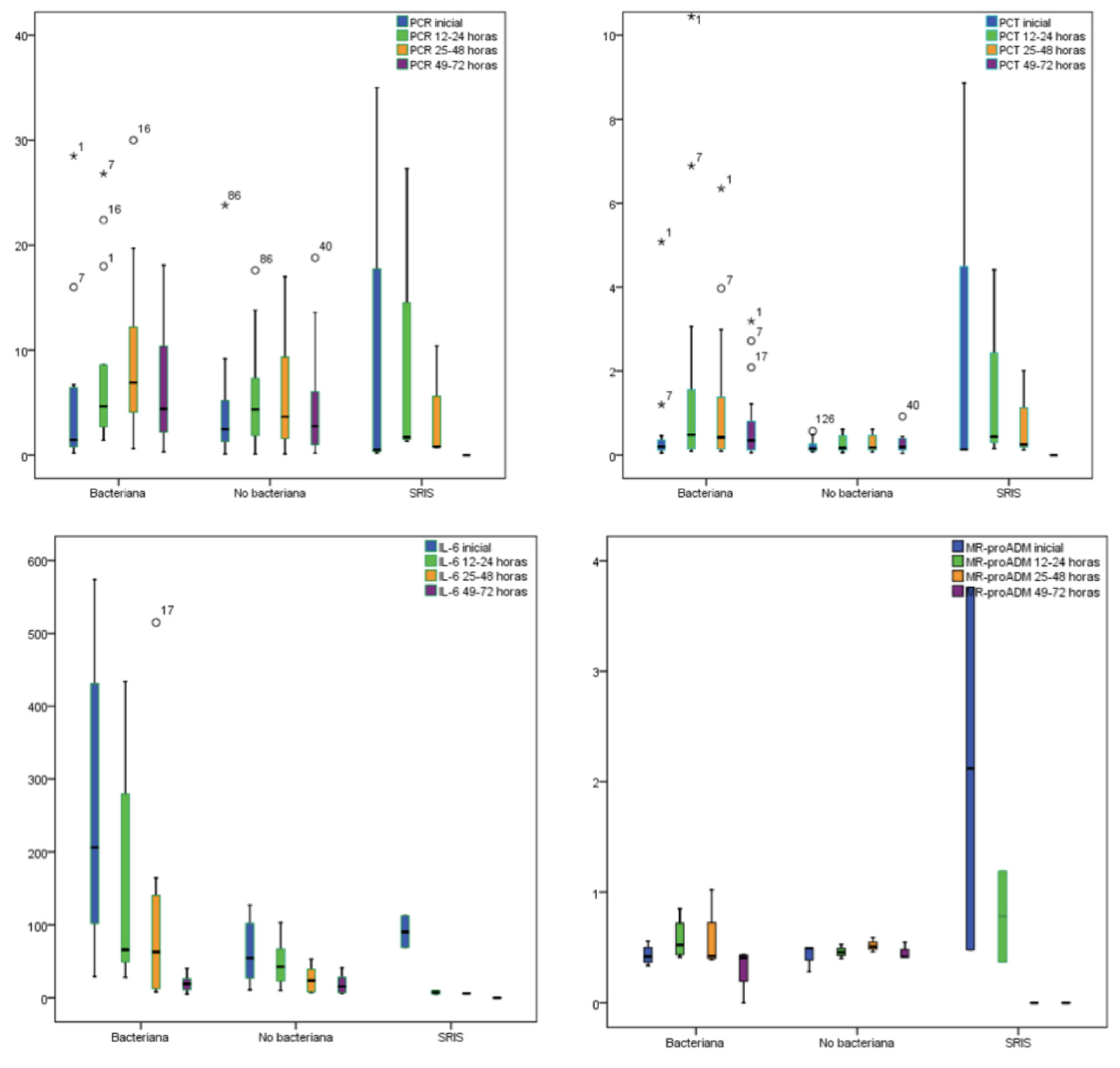
| Biomarkers levels (Baseline) | Bacterial infections median (IQR) | Non bacterial infections median (IQR) | SIRS median (IQR) | Value p median (IQR) |
| CRP mg/dL | 1.45 (4.7) | 1.6 (3.6) | 1.4 (5.7) | 0.950 |
| PCT ng/mL | 0.15 (0.15) | 0.13 (0.12) | 0.32 (4.49) | 0.150 |
| IL-6 pg/mL | * 78 (152.5) | 52 (55.5) | X 112 (4143) | <0.005 |
| MR-proADM nmol/L | 0.47 (0.16) | 0.47 (0.12) | 0.75 (2.67) | 0.060 |
| Biomarkers levels (12–24 h) | Bacterial infections median (IQR) | Non bacterial infections median (IQR) | SIRS median (IQR) | Value p median (IQR) |
| CRP mg/dL | 4.45 (5.9) | 4.3 (9.9) | 12.95 (25.13) | 0.842 |
| PCT ng/mL | 0.35 (2.14) | 0.2 (0.33) | 2.43 (14.85) | 0.089 |
| IL-6 pg/mL | *X 75.5 (163) | 33.5 (51.25) | 14 (17) | <0.005 |
| MR-proADM nmol/L | 0.46 (0.27) | 0.46 (0.19) | 0.9 (-) | 0.378 |
| Biomarkers levels (25–48 h) | Bacterial infections median (IQR) | Non bacterial infections median (IQR) | SIRS median (IQR) | Value p median (IQR) |
| CRP mg/dL | 5.20 (8.3) | 4,7 (9.5) | 3.45 (8.6) | 0.360 |
| PCT ng/mL | 0.4 (0.74) | 0.21 (0.43) | 1.13 (8.99) | 0.423 |
| IL-6 pg/mL | 29 (92) | 17 (35.25) | 6 (-) | 0.540 |
| MR-proADM nmol/L | 0.43 (0.34) | 0.47 (0.20) | 0.93 (-) | 0.333 |
| Biomarkers levels (49–72 h) | Bacterial infections median (IQR) | Non bacterial infections median (IQR) | SIRS median (IQR) | Value p median (IQR) |
| CRP mg/dL | 3.70 (7.20) | 3.10 (7.10) | - | 0.935 |
| PCT ng/mL | 0.31 (0.59) | 0.18 (0.27) | - | 0.665 |
| IL-6 pg/mL | 14 (15) | 15.5 (30.75) | - | 1 |
| MR-proADM nmol/L | 0.41 (0.34) | 0.41 (0.21) | - | 0.773 |
| Biomarkers Levels (Baseline) | Neutrophils | Median | IQR | Value p |
|---|---|---|---|---|
| CRP (mg/dL) | >1500/μL | 1.4 | 3.20 | 0.373 |
| 500–1500/μL | 2.6 | 5.20 | ||
| <500/μL | 1.9 | 4.40 | ||
| PCT (ng/mL) | >1500/μL | 0.12 | 0.13 | 0.067 |
| 500–1500/μL | 0.20 | 0.13 | ||
| <500/μL | 0.15 | 0.15 | ||
| IL-6 (pg/mL) | >1500/μL | 59.5 | 85.5 | 0.411 |
| 500–1500/μL | 41.5 | 105.25 | ||
| <500/μL | 64 | 60 | ||
| MR-proADM (nmol/L) | >1500/μL | 0.48 | 0.22 | 0.252 |
| 500–1500/μL | 0.52 | 0.18 | ||
| <500/μL | 0.46 | 0.13 |
| Biomarkers Cutoff Value | Highest Level Point of Each BM | Bacterial Infections | Non Bacterial Infections | SIRS | Value p |
|---|---|---|---|---|---|
| PCR > 4 mg/dL | 25–48 h | 73.6% | 53.8% | 50% | 0.343 |
| PCT > 0.5 ng/mL | 12–24 h | 36.3% | 23.2% | 50% | 0.267 |
| Il-6 > 85 pg/mL | Baseline | 47.6% | 22.2% | * 71.4% | 0.007 |
| MR-proADM > 0.5 nmol/L | Baseline | 42.1% | 31.8% | 80% | 0.115 |
| Biomarkers (Baseline) | PCR (Value of p) | PCT (Value of p) | IL-6 (Value of p) | MR-proADM (Value of p) |
|---|---|---|---|---|
| CRP | - | <0.001 | 0.043 | 0.261 |
| PCT | <0.001 | - | 0.045 | <0.001 |
| IL-6 | 0.043 | 0.045 | - | <0.001 |
| MR-proADM | 0.261 | <0.001 | <0.001 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lucio Delgado, A.; Villegas Rubio, J.A.; Rey Galan, C.; Prieto García, B.; González Expósito, M.d.l.R.; Solís Sánchez, G. Biomarkers and Fever in Children with Cancer: Kinetics and Levels According to Final Diagnosis. Children 2021, 8, 1027. https://doi.org/10.3390/children8111027
de Lucio Delgado A, Villegas Rubio JA, Rey Galan C, Prieto García B, González Expósito MdlR, Solís Sánchez G. Biomarkers and Fever in Children with Cancer: Kinetics and Levels According to Final Diagnosis. Children. 2021; 8(11):1027. https://doi.org/10.3390/children8111027
Chicago/Turabian Stylede Lucio Delgado, Ana, Jose Antonio Villegas Rubio, Corsino Rey Galan, Belen Prieto García, Maria de los Reyes González Expósito, and Gonzalo Solís Sánchez. 2021. "Biomarkers and Fever in Children with Cancer: Kinetics and Levels According to Final Diagnosis" Children 8, no. 11: 1027. https://doi.org/10.3390/children8111027
APA Stylede Lucio Delgado, A., Villegas Rubio, J. A., Rey Galan, C., Prieto García, B., González Expósito, M. d. l. R., & Solís Sánchez, G. (2021). Biomarkers and Fever in Children with Cancer: Kinetics and Levels According to Final Diagnosis. Children, 8(11), 1027. https://doi.org/10.3390/children8111027






