Using Body Composition Groups to Identify Children and Adolescents at Risk of Dyslipidemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Measurements
2.3. Definition of Variables
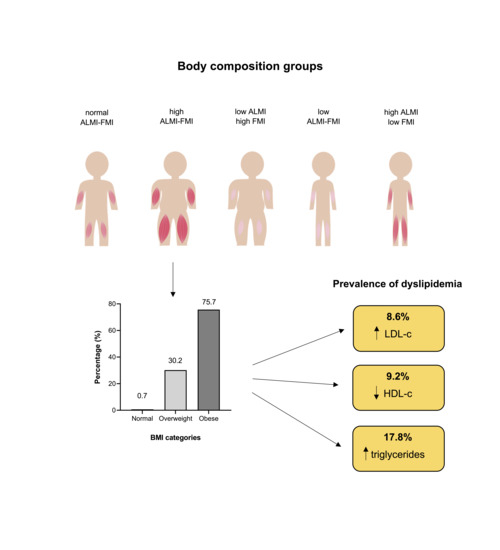
2.4. Body Composition Groups
2.5. Blood Samples
2.6. Questionnaires
2.7. Statistics
3. Results
3.1. Serum Lipid Profiles
3.2. Body Composition Group Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Singh, G.M.; Danaei, G.; Farzadfar, F.; Stevens, G.A.; Woodward, M.; Wormser, D.; Kaptoge, S.; Whitlock, G.; Qiao, Q.; Lewington, S. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: A pooled analysis. PLoS ONE 2013, 8, e65174. [Google Scholar]
- Anandacoomarasamy, A.; Caterson, I.; Sambrook, P.; Fransen, M.; March, L. The impact of obesity on the musculoskeletal system. Int. J. Obes. 2008, 32, 211–222. [Google Scholar] [CrossRef]
- Forno, E.; Han, Y.-Y.; Libman, I.M.; Muzumdar, R.H.; Celedón, J.C. Adiposity and Asthma in a Nationwide Study of Children and Adults in the United States. Ann. Am. Thorac. Soc. 2017, 15, 322–330. [Google Scholar] [CrossRef]
- Prentice, A.M.; Jebb, S.A. Beyond body mass index. Obes. Rev. 2001, 2, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Forbes, G.B. Lean Body mass and fat in obese children. Pediatrics 1964, 34, 308–314. [Google Scholar] [PubMed]
- Van Aller, C.; Lara, J.; Stephan, B.C.M.; Donini, L.M.; Heymsfield, S.; Katzmarzyk, P.T.; Wells, J.C.K.; Prado, C.M.; Siervo, M. Sarcopenic obesity and overall mortality: Results from the application of novel models of body composition phenotypes to the National Health and Nutrition Examination Survey 1999. Clin. Nutr. 2019, 38, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Orsso, C.E.; Tibaes, J.R.B.; Oliveira, C.L.P.; Rubin, D.A.; Field, C.J.; Heymsfield, S.B.; Prado, C.M.; Haqq, A.M. Low muscle mass and strength in pediatrics patients: Why should we care? Clin. Nutr. 2019, 38, 2002–2015. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Pinto, A.; Giusti, A.M.; Lenzi, A.; Poggiogalle, E. Obesity or BMI Paradox? Beneath the Tip of the Iceberg. Front. Nutr. 2020, 7, 53. [Google Scholar] [PubMed]
- Emmett, P.M.; Jones, L.R. Diet, growth, and obesity development throughout childhood in the Avon Longitudinal Study of Parents and Children. Nutr. Rev. 2015, 73, 175–206. [Google Scholar] [CrossRef]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef]
- Mameli, C.; Mazzantini, S.; Zuccotti, G.V. Nutrition in the first 1000 days: The origin of childhood obesity. Int. J. Environ. Res. Public Health 2016, 13, 838. [Google Scholar] [CrossRef] [PubMed]
- Origin of atherosclerosis in childhood and adolescence. Am. J. Clin. Nutr. 2000, 72, 1307s–1315s. [CrossRef] [PubMed]
- Nadeau, K.J.; Anderson, B.J.; Berg, E.G.; Chiang, J.L.; Chou, H.; Copeland, K.C.; Hannon, T.S.; Huang, T.T.-K.; Lynch, J.L.; Powell, J.; et al. Youth-Onset Type 2 Diabetes Consensus Report: Current Status, Challenges, and Priorities. Diabetes Care 2016, 39, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Forsén, T.; Eriksson, J.; Tuomilehto, J.; Reunanen, A.; Osmond, C.; Barker, D. The Fetal and Childhood Growth of Persons Who Develop Type 2 Diabetes. Ann. Intern. Med. 2000, 133, 176–182. [Google Scholar] [CrossRef] [PubMed]
- 1Svanes, C.; Sunyer, J.; Plana, E.; Dharmage, S.; Heinrich, J.; Jarvis, D.; de Marco, R.; Norbäck, D.; Raherison, C.; Villani, S.; et al. Early life origins of chronic obstructive pulmonary disease. Thorax 2010, 65, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Juonala, M.; Wu, F.; Sinaiko, A.; Woo, J.G.; Urbina, E.M.; Jacobs, D.; Steinberger, J.; Prineas, R.; Koskinen, J.; Sabin, M.A.; et al. Non-HDL Cholesterol Levels in Childhood and Carotid Intima-Media Thickness in Adulthood. Pediatrics 2020, 145, e20192114. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, C.G.; Venn, A.; Thomson, R.; Juonala, M.; Srinivasan, S.R.; Viikari, J.S.A.; Berenson, G.S.; Dwyer, T.; Raitakari, O.T. The Association of Pediatric Low- and High-Density Lipoprotein Cholesterol Dyslipidemia Classifications and Change in Dyslipidemia Status With Carotid Intima-Media Thickness in Adulthood: Evidence From the Cardiovascular Risk in Young Finns Study, the Bog. J. Am. Coll. Cardiol. 2009, 53, 860–869. [Google Scholar] [CrossRef]
- Breyer-Kohansal, R.; Hartl, S.; Burghuber, O.C.; Urban, M.; Schrott, A.; Agusti, A.; Sigsgaard, T.; Vogelmeier, C.; Wouters, E.; Studnicka, M.; et al. The LEAD (Lung, Heart, Social, Body) Study: Objectives, Methodology, and External Validity of the Population-Based Cohort Study. J. Epidemiol. 2019, 29, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Ofenheimer, A.; Breyer-Kohansal, R.; Hartl, S.; Burghuber, O.C.; Krach, F.; Schrott, A.; Wouters, E.F.M.; Franssen, F.M.E.; Breyer, M.-K. Reference values of body composition parameters and visceral adipose tissue (VAT) by DXA in adults aged 18–81 years—results from the LEAD cohort. Eur. J. Clin. Nutr. 2020, 74, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Orsso, C.E.; Tibaes, J.R.B.; Rubin, D.A.; Field, C.J.; Heymsfield, S.B.; Prado, C.M.; Haqq, A.M. Metabolic implications of low muscle mass in the pediatric population: A critical review. Metab.-Clin. Exp. 2019, 99, 102–112. [Google Scholar] [CrossRef]
- Peterson, C.M.; Su, H.; Thomas, D.M.; Heo, M.; Golnabi, A.H.; Pietrobelli, A.; Heymsfield, S.B. Tri-Ponderal Mass Index vs Body Mass Index in Estimating Body Fat During Adolescence. JAMA Pediatr. 2017, 171, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.F. Measures of adiposity: The inappropriate use of the fat mass index. Int. J. Obes. 2010, 34, 213. [Google Scholar] [CrossRef]
- Ofenheimer, A.; Breyer-Kohansal, R.; Hartl, S.; Burghuber, O.C.; Krach, F.; Schrott, A.; Franssen, F.M.E.; Wouters, E.F.M.; Breyer, M.-K. Reference charts for body composition parameters by dual-energy X-ray absorptiometry in European children and adolescents aged 6 to 18 years—Results from the Austrian LEAD (Lung, hEart, sociAl, boDy) cohort. Pediatr. Obes. 2020, 16, e12695. [Google Scholar] [CrossRef] [PubMed]
- Onis, M.D.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128, S213. [CrossRef] [PubMed]
- Prado, C.M.M.; Siervo, M.; Mire, E.; Heymsfield, S.B.; Stephan, B.C.M.; Broyles, S.; Smith, S.R.; Wells, J.C.K.; Katzmarzyk, P.T. A population-based approach to define body-composition phenotypes. Am. J. Clin. Nutr. 2014, 99, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Engelen, M.P.K.J.; Schroder, R.; Van der Hoorn, K.; Deutz, N.E.P.; Com, G. Use of body mass index percentile to identify fat-free mass depletion in children with cystic fibrosis. Clin. Nutr. 2012, 31, 927–933. [Google Scholar] [CrossRef]
- Freedman, D.S.; Wang, J.; Maynard, L.M.; Thornton, J.C.; Mei, Z.; Pierson, R.N.; Dietz, W.H.; Horlick, M. Relation of BMI to fat and fat-free mass among children and adolescents. Int. J. Obes. 2005, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bichteler, A.; Gershoff, E.T. Identification of Children’s BMI Trajectories and Prediction from Weight Gain in Infancy. Obesity 2018, 26, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Wibaek, R.; Vistisen, D.; Girma, T.; Admassu, B.; Abera, M.; Abdissa, A.; Mudie, K.; Kæstel, P.; Jørgensen, M.E.; Wells, J.C.K.; et al. Body mass index trajectories in early childhood in relation to cardiometabolic risk profile and body composition at 5 years of age. Am. J. Clin. Nutr. 2019, 110, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Aris, I.M.; Oken, E. Childhood Adiposity Trajectories: Discerning Order Amongst the Chaos. Am. J. Clin. Nutr. 2019, 110, 1049–1050. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.S.; Chumlea, W.M.C.; Roche, A.F.; Siervogel, R.M. Age-and maturity-related changes in body composition during adolescence into adulthood: The Fels Longitudinal Study. Appl. Radiat. Isot. 1998, 49, 581–585. [Google Scholar] [CrossRef]
- Chomtho, S.; Wells, J.C.K.; Williams, J.E.; Lucas, A.; Fewtrell, M.S. Associations between birth weight and later body composition: Evidence from the 4-component model. Am. J. Clin. Nutr. 2008, 88, 1040–1048. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Müller, M.J. Identification of skeletal muscle mass depletion across age and BMI groups in health and disease—there is need for a unified definition. Int. J. Obes. 2015, 39, 379–386. [Google Scholar] [CrossRef]
- Kim, S.G.; dong Ko, K.; Hwang, I.C.; Suh, H.S.; Kay, S.; Caterson, I.; Kim, K.K. Relationship between indices of obesity obtained by anthropometry and dual-energy X-ray absorptiometry: The Fourth and Fifth Korea National Health and Nutrition Examination Survey (KNHANES IV and V, 2008–2011). Obes. Res. Clin. Pract. 2015, 9, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.D.; Zhang, P.; Saltarelli, W.A.; Visich, P.S.; Gordon, P.M. Low Muscle Strength Thresholds for the Detection of Cardiometabolic Risk in Adolescents. Am. J. Prev. Med. 2016, 50, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.J.; Metcalf, B.S.; Jeffery, A.N.; Voss, L.D.; Wilkin, T.J. Does lean rather than fat mass provide the link between birth weight, BMI, and metabolic risk? EarlyBird 23. Pediatr. Diabetes 2006, 7, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Blakeley, C.E.; Van Rompay, M.I.; Schultz, N.S.; Sacheck, J.M. Relationship between muscle strength and dyslipidemia, serum 25 (OH) D, and weight status among diverse schoolchildren: A cross-sectional analysis. BMC Pediatr. 2018, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Meredith-Jones, K.; Haszard, J.; Moir, C.; Heath, A.-L.; Lawrence, J.; Galland, B.; Taylor, B.; Gray, A.; Sayers, R.; Taylor, R. Physical activity and inactivity trajectories associated with body composition in pre-schoolers. Int. J. Obes. 2018, 42, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F.; Puyau, M.R.; Wilson, T.A.; Liu, Y.; Wong, W.W.; Adolph, A.L.; Zakeri, I.F. Role of physical activity and sleep duration in growth and body composition of preschool-aged children. Obesity 2016, 24, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- McCormack, L.; Meendering, J.; Specker, B.; Binkley, T. Associations between sedentary time, physical activity, and dual-energy X-ray absorptiometry measures of total body, android, and gynoid fat mass in children. J. Clin. Densitom. 2016, 19, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Jen, V.; Karagounis, L.G.; Jaddoe, V.W.V.; Franco, O.H.; Voortman, T. Dietary protein intake in school-age children and detailed measures of body composition: The Generation R Study. Int. J. Obes. 2018, 42, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Voortman, T.; Braun, K.V.E.; Kiefte-de Jong, J.C.; Jaddoe, V.W.V.; Franco, O.H.; van den Hooven, E.H. Protein intake in early childhood and body composition at the age of 6 years: The Generation R Study. Int. J. Obes. 2016, 40, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Rolland-Cachera, M.F.; Deheeger, M.; Maillot, M.; Bellisle, F. Early adiposity rebound: Causes and consequences for obesity in children and adults. Int. J. Obes. 2006, 30, S11–S17. [Google Scholar] [CrossRef]
- Assmann, K.E.; Joslowski, G.; Buyken, A.E.; Cheng, G.; Remer, T.; Kroke, A.; Günther, A.L.B. Prospective association of protein intake during puberty with body composition in young adulthood. Obesity 2013, 21, E782–E789. [Google Scholar] [CrossRef] [PubMed]
- Burrows, R.; Correa-Burrows, P.; Reyes, M.; Blanco, E.; Albala, C.; Gahagan, S. Low muscle mass is associated with cardiometabolic risk regardless of nutritional status in adolescents: A cross-sectional study in a Chilean birth cohort. Pediatr. Diabetes 2017, 18, 895–902. [Google Scholar] [CrossRef]
- Williams, D.P.; Going, S.B.; Lohman, T.G.; Harsha, D.W.; Srinivasan, S.R.; Webber, L.S.; Berenson, G.S. Body fatness and risk for elevated blood pressure, total cholesterol, and serum lipoprotein ratios in children and adolescents. Am. J. Public Health 1992, 82, 358–363. [Google Scholar] [CrossRef]
- Choi, J.W.; Pai, S.H.; Kim, S.K. Associations between total body fat and serum lipid concentrations in obese human adolescents. Ann. Clin. Lab. Sci. 2002, 32, 271–278. [Google Scholar]
- Lamb, M.M.; Ogden, C.L.; Carroll, M.D.; Lacher, D.A.; Flegal, K.M. Association of body fat percentage with lipid concentrations in children and adolescents: United States, 1999–20041–4. Am. J. Clin. Nutr. 2011, 94, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Duran, I.; Martakis, K.; Schafmeyer, L.; Jackels, M.; Rehberg, M.; Schoenau, E. Inverse association of high-density lipoprotein cholesterol concentration with muscle mass in children. Child. Obes. 2019, 15, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Pietrobelli, A.; Lee, R.C.; Capristo, E.; Deckelbaum, R.J.; Heymsfield, S.B. An independent, inverse association of high-density-lipoprotein-cholesterol concentration with nonadipose body mass. Am. J. Clin. Nutr. 1999, 69, 614–620. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Borga, M.; West, J.; Bell, J.D.; Harvey, N.C.; Romu, T.; Heymsfield, S.B.; Leinhard, O.D. Advanced body composition assessment: From body mass index to body composition profiling. J. Investig. Med. 2018, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Heard-Lipsmeyer, M.E.; Hull, H.; Sims, C.R.; Cleves, M.A.; Andres, A. Evaluating body composition in infancy and childhood: A comparison between 4C, QMR, DXA, and ADP. Pediatr. Obes. 2020, 15, e12617. [Google Scholar] [CrossRef] [PubMed]



| Males n (%) | Females n (%) | Overall n (%) | |
|---|---|---|---|
| FMI (kg/m2.5) | |||
| normal | 348 (46.8%) | 307 (54.1%) | 655 (47.0%) |
| low | 206 (27.7%) | 177 (27.2%) | 383 (27.5%) |
| high | 190 (25.5%) | 166 (25.5%) | 356 (25.5%) |
| ALMI (kg/m3.5) | |||
| normal | 361 (48.5%) | 333 (51.2%) | 694 (49.8%) |
| low | 185 (24.9%) | 159 (24.5%) | 344 (24.7%) |
| high | 198 (26.6%) | 158 (24.3%) | 356 (25.5%) |
| ALMI-FMI groups | |||
| normal ALMI-FMI | 535 (71.9%) | 459 (70.6%) | 994 (71.3%) |
| low ALMI-FMI | 72 (9.7%) | 74 (11.4%) | 146 (10.5%) |
| high ALMI-FMI | 79 (10.6%) | 84 (12.9%) | 163 (11.7%) |
| low ALMI-high FMI | 21 (2.8%) | 14 (2.2%) | 35 (2.5%) |
| high ALMI-low FMI | 37 (5.0%) | 19 (2.9%) | 56 (4.0%) |
| BMI category | |||
| extreme thinness | 16 (2.2%) | 9 (1.4%) | 25 (1.8%) |
| thinness | 83 (11.2%) | 93 (14.3%) | 176 (12.6%) |
| normal | 443 (59.5%) | 397 (61.1%) | 840 (60.3%) |
| overweight | 131 (17.6%) | 111 (17.1%) | 242 (17.4%) |
| obesity | 71 (9.5%) | 40 (6.2%) | 111 (8.0%) |
| total | 744 | 650 | 1394 |
| HDL-c [mg/dL] | HDL-c z-Scores | LDL-c [mg/dL] | LDL-c z-Scores | Triglycerides [mg/dL] | Triglycerides z-Scores | |
|---|---|---|---|---|---|---|
| FMI [kg/m2.5] | ||||||
| normal | 61.0 (53.0, 70.0) | 0.0 (−0.6, 0.6) | 86.6 (70.4, 102.5) | 0.0 (−0.7, 0.6) | 63.0 (48.0, 86.0) | 0.0 (−0.7, 0.6) |
| low | 65.0 (54.0, 75.0) ■ | 0.3 (−0.3, 1.0) ■ | 82.4 (68.3, 97.3) | −0.2 (−0.8, 0.4) | 57.0 (47.0, 77.0) | −0.3 (−0.8, 0.4) |
| high | 54.5 (46.0, 64.0) ■ † | −0.4 (−1.2, 0.2) ■ † | 92.8 (77.3, 111.3) ■ † | 0.3 (−0.4, 0.9) ■ † | 74.0 (56.0, 104.3) ■ † | 0.3 (−0.3, 1.0) ■ † |
| ALMI [kg/m3.5] | ||||||
| normal | 59.0 (51.0, 70.0) | 0.0 (−0.7, 0.6) | 86.6 (71.9, 102.0) | 0.0 (−0.6, 0.6) | 62.0 (49.0, 86.0) | 0.0 (−0.7, 0.6) |
| low | 64.0 (54.8, 72.0) ■ | 0.2 (−0.4. 0.8) ■ | 84.2 (69.9, 102.9) | −0.1 (−0.8, 0.6) | 62.0 (48.0, 86.3) | −0.1 (−0.7, 0.6) |
| high | 58.0 (48.8, 69.0) † | −0.1 (−0.9, 0.6) † | 90.2 (74.0, 108.4) | 0.1 (−0.6, 0.8) | 66.0 (51.0, 93.3) | 0.1 (−0.6, 0.9) |
| ALMI-FMI groups | ||||||
| normal ALMI- FMI | 60.0 (52.0, 70.0) | 0.0 (−0.6, 0.6) | 86.7 (71.4, 102.0) | −0.0 (−0.6, 0.6) | 62.0 (49.0, 87.8) | −0.0 (−0.7, 0.6) |
| low ALMI-FMI | 66.0 (57.0, 75.8)* | 0.4 (−0.3, 1.0)* | 80.8 (66.9, 97.8) | −0.2 (−0.8, 0.5) | 58.5 (47.0, 78.0) | −0.3 (−0.8, 0.4) |
| high ALMI-FMI | 53.0 (45.0, 62.0) *▲● | −0.5 (−1.2, 0.0) *▲● | 97.4 (77.6, 113.7) *▲ | 0.4 (−0.5, 1.0) *▲ | 76.0 (57.0, 106.0) *▲● | 0.4 (−0.3, 1.1) *▲● |
| low ALMI-high FMI | 60.0 (50.0, 64.5) | −0.2 (−0.8, 0.5) | 92.0 (76.1, 112.1) | 0.4 (−0.4, 1.0) | 70.0 (54.0, 91.0) | 0.3 (−0.4, 0.7) |
| high ALMI-low FMI | 66.0 (53.8, 77.0) | 0.4 (−0.4, 1.0) | 84.3 (70.8, 96.2) | -0.1 (−0.7, 0.5) | 57.5 (46.8, 69.2) | −0.4 (−0.7, 0.3) |
| Normal ALMI FMI | Low ALMI FMI | High ALMI FMI | Low ALMI High FMI | High ALMI Low FMI | |
|---|---|---|---|---|---|
| Demographics | |||||
| age [years] | 10.8 (8.3, 14.6) | 10.9 (8.7, 14.5) | 10.8 (8.7, 14.5) | 9.9 (8.5, 15.1) | 10.4 (8.2, 16.1) |
| sex [%females] | 46.2% | 50.7% | 51.5% | 40.0% | 33.9% |
| height [cm] | 146.0 (132.0, 164.0) | 151.5 (133.0,168.0) | 148.0 (135.0,163.0) | 146.0 (136.0,166.5) | 140.5 (127.0,168.3) |
| weight [kg] | 39.0 (28.0, 55.0) | 33.5 (24.0, 46.8) * | 54.0 (38.0, 71.0) * | 43.0 (32.5, 60.0) | 33.5 (25.8, 56.5) |
| waist circumference [cm] | 65.5 (59.0, 74.5) | 60.5 (55.6, 68.5) * | 80.5 (70.8, 90.3) * | 76.0 (66.3, 80.5) * | 60.8 (56.0, 68.6) |
| hand grip strength [kg] | 17.5 (12.9, 26.2) | 18.2 (12.5, 26.5) | 19.5 (14.2, 27.2) | 13.2 (11.4, 21.9) | 19.6 (13.9, 32.2) |
| Socio-economic status | |||||
| low | 11.8% | 8.2% | 21.5% | 8.6% | 10.7% |
| normal | 44.3% | 39.7% | 47.2% | 68.6% | 39.3% |
| high | 43.9% | 50.7% | 31.3% | 22.9% | 50.0% |
| Early life risk factors | |||||
| birthweight [kg] | 3.3 (2.8, 3.7) | 3.2 (2.4, 3.6) | 3.3 (2.7, 3.7) | 3.3 (1.0, 3.9) | 3.2 (2.3, 3.5) |
| preterm birth [%] | 9.2% | 15.1% | 8.0% | 11.4% | 14.3% |
| low birthweight | 20.7% | 26.0% | 22.1% | 34.3% | 28.6% |
| breast feeding ever | 89.5% | 91.1% | 82.8% | 82.9% | 96.4% |
| Smoke exposure | |||||
| second-hand smoking | 17.5% | 15.1% | 25.3% | 25.7% | 3.6% |
| maternal smoking | |||||
| prior pregnancy | 38.2% | 34.2% | 44.2% | 65.7% | 26.8% |
| during pregnancy | 9.3% | 4.1% | 14.7% | 22.9% | 3.6% |
| after pregnancy | 11.9% | 8.9% | 16.6% | 22.9% | 5.4% |
| Lifestyle factors | |||||
| physical activity [minutes/day] | 79.3 (49.8, 110.7) | 70.7 (43.2, 103.6) | 62.1 (41.4, 99.3) | 70.7 (44.3, 98.3) | 95.0 (77.9, 120.5) |
| physical activity ≥60 minutes/day | 67.8% | 58.9% | 54.0% * | 68.6% | 91.1% * |
| healthy nutrition | 26.9% | 26.0% | 28.2% | 25.7% | 26.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ofenheimer, A.; Breyer-Kohansal, R.; Hartl, S.; Burghuber, O.C.; Krach, F.; Franssen, F.M.E.; Wouters, E.F.M.; Breyer, M.-K. Using Body Composition Groups to Identify Children and Adolescents at Risk of Dyslipidemia. Children 2021, 8, 1047. https://doi.org/10.3390/children8111047
Ofenheimer A, Breyer-Kohansal R, Hartl S, Burghuber OC, Krach F, Franssen FME, Wouters EFM, Breyer M-K. Using Body Composition Groups to Identify Children and Adolescents at Risk of Dyslipidemia. Children. 2021; 8(11):1047. https://doi.org/10.3390/children8111047
Chicago/Turabian StyleOfenheimer, Alina, Robab Breyer-Kohansal, Sylvia Hartl, Otto C. Burghuber, Florian Krach, Frits M. E. Franssen, Emiel F. M. Wouters, and Marie-Kathrin Breyer. 2021. "Using Body Composition Groups to Identify Children and Adolescents at Risk of Dyslipidemia" Children 8, no. 11: 1047. https://doi.org/10.3390/children8111047
APA StyleOfenheimer, A., Breyer-Kohansal, R., Hartl, S., Burghuber, O. C., Krach, F., Franssen, F. M. E., Wouters, E. F. M., & Breyer, M.-K. (2021). Using Body Composition Groups to Identify Children and Adolescents at Risk of Dyslipidemia. Children, 8(11), 1047. https://doi.org/10.3390/children8111047