Spontaneous Closure of the Arterial Duct after Transcatheter Closure Attempt in Preterm Infants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Procedure
2.3. Statistical Analysis
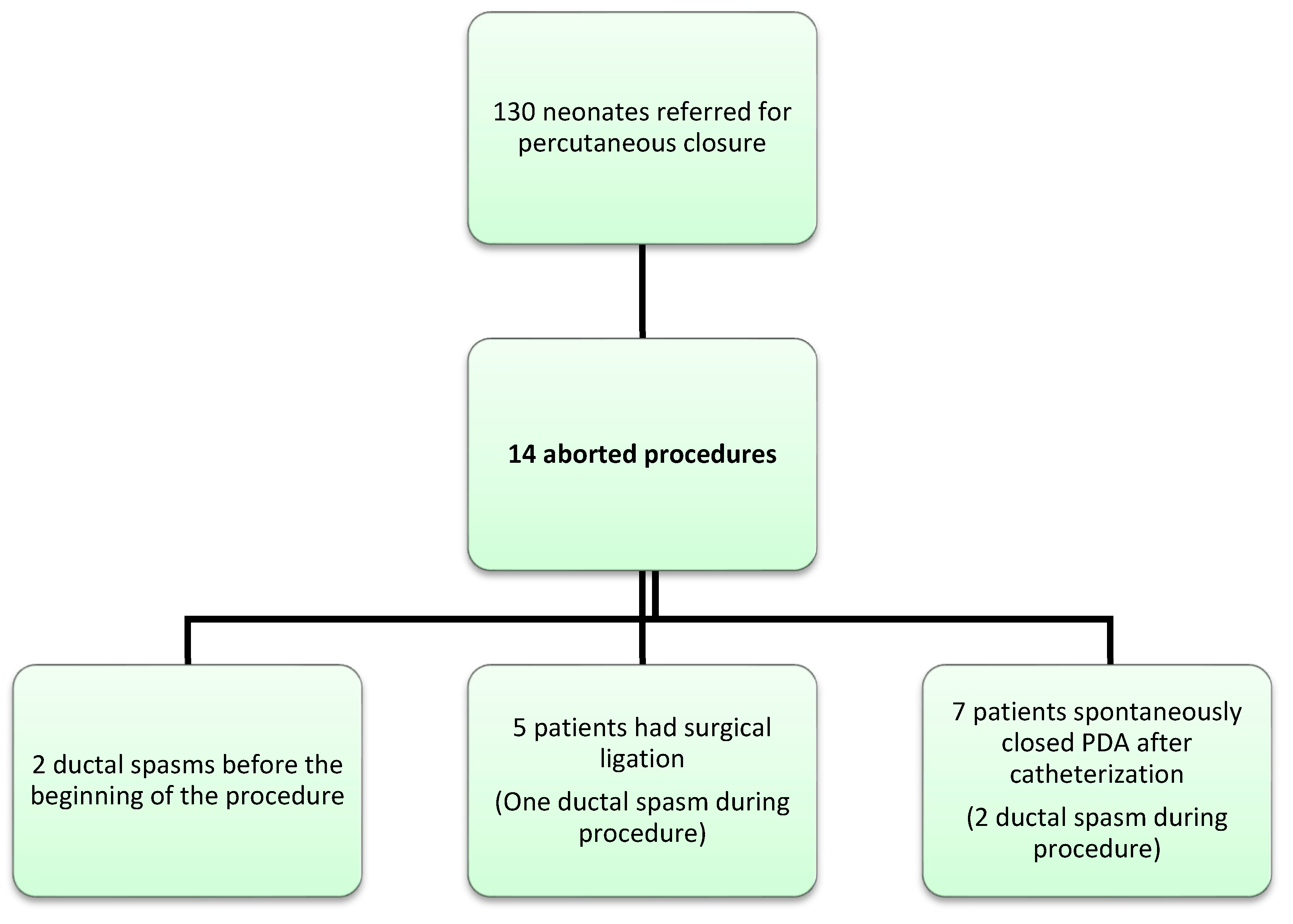
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hamrick, S.E.; Sallmon, H.; Rose, A.T.; Porras, D.; Shelton, E.L.; Reese, J.; Hansmann, G. Patent Ductus Arteriosus of the Preterm Infant. Pediatrics 2020, 146, e20201209. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.I.; Chang, Y.S.; Kim, J.; Choi, J.H.; Ahn, S.Y.; Park, W.S. Natural evolution of ductus arteriosus with noninterventional conservative management in extremely preterm infants born at 23-28 weeks of gestation. PLoS ONE 2019, 14, e0212256. [Google Scholar] [CrossRef] [PubMed]
- Sathanandam, S.; Balduf, K.; Chilakala, S.; Washington, K.; Rn, K.A.; Knott-Craig, C.; Waller, B.R.; Philip, R. Role of Transcatheter patent ductus arteriosus closure in extremely low birth weight infants. Catheter. Cardiovasc. Interv. 2018, 93, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Zahn, E.M.; Peck, D.; Phillips, A.; Nevin, P.; Basaker, K.; Simmons, C.; McRae, M.; Early, T.; Garg, R. Transcatheter Closure of Patent Ductus Arteriosus in Extremely Premature Newborns: Early Results and Midterm Follow-Up. JACC Cardiovasc. Interv. 2016, 9, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Scerbo, D.; Cua, C.L.; Rivera, B.K.; Marzec, L.C.; Smith, C.V.; Slaughter, J.L.; Berman, D.P.; Backes, C.H. Percutaneous Closure of the Patent Ductus Arteriosus in Very-Low-Weight Infants. NeoReviews 2020, 21, e469–e478. [Google Scholar] [CrossRef] [PubMed]
- Sathanandam, S.; Agrawal, H.; Chilakala, S.; Johnson, J.; Rn, K.A.; Knott-Craig, C.; Waller, B.R.; Philip, R. Can transcatheter PDA closure be performed in neonates ≤1000 grams? The Memphis experience. Congenit. Heart Dis. 2019, 14, 79–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malekzadeh-Milani, S.; Akhavi, A.; Douchin, S.; Dauphin, C.; Chalard, A.; Mauran, P.; Bouvaist, H.; Bonnet, D.; Boudjemline, Y. Percutaneous closure of patent ductus arteriosus in premature infants: A French national survey. Catheter. Cardiovasc. Interv. 2019, 95, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Meot, M.; Gaudin, R.; Szezepanski, I.; Bajolle, F.; Bonnet, D.; Malekzadeh-Milani, S. Transcatheter patent arterial duct closure in premature infants: A new technique to ease access to the patent arterial duct, with particular benefit for the tricuspid valve. Arch. Cardiovasc. Dis. 2021, 114, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Markush, D.; Tsing, J.C.; Gupta, S.; Berndsen, N.C.; Radville, G.; Garg, R.; Zahn, E.M.; Almeida-Jones, M. Fate of the Left Pulmonary Artery and Thoracic Aorta After Transcatheter Patent Ductus Arteriosus Closure in Low Birth Weight Premature Infants. Pediatr. Cardiol. 2021, 42, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.H.; Giesinger, R.E.; Rivera, B.K.; Berman, D.P.; Smith, C.V.; Cua, C.L.; Kelleher, K.J.; McNamara, P.J.; Slaughter, J.L. Percutaneous Closure of the Patent Ductus Arteriosus in Very Low Weight Infants: Considerations Following US Food and Drug Administration Approval of a Novel Device. J. Pediatr. 2019, 213, 218–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathanandam, S.; Gutfinger, D.; Morray, B.; Berman, D.; Gillespie, M.; Forbes, T.; Johnson, J.N.; Garg, R.; Malekzadeh-Milani, S.; Fraisse, A.; et al. Consensus Guidelines for the Prevention and Management of Periprocedural Complications of Transcatheter Patent Ductus Arteriosus Closure with the Amplatzer Piccolo Occluder in Extremely Low Birth Weight Infants. Pediatr. Cardiol. 2021, 42, 1258–1274. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, A.; Nagasawa, H.; Yamamoto, Y.; Tatebayashi, K.; Suzuki, H.; Yamada, K.; Arai, M.; Kohno, Y. Clinical aspects of very-low-birthweight infants showing reopening of ductus arteriosus. Pediatr. Int. 2011, 53, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Halil, H.; Buyuktiryaki, M.; Atay, F.Y.; Oncel, M.Y.; Uras, N. Reopening of the ductus arteriosus in preterm infants; Clinical aspects and subsequent consequences. J. Neonatal-Perinat. Med. 2018, 11, 273–279. [Google Scholar] [CrossRef] [PubMed]
- De Decker, R.; Comitis, G.; Thomas, J.; van der Merwe, E.; Lawrenson, J. A novel approach to ductal spasm during percutaneous device occlusion of patent ductus arteriosus. Cardiol. Young 2016, 26, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Batlivala, S.P.; Glatz, A.C.; Gillespie, M.J.; Dori, Y.; Rome, J.J. Ductal spasm during performance of transcatheter ductal occlusion. Catheter. Cardiovasc. Interv. 2014, 83, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Lozier, J.S.; Cowley, C.G. Reactivity of the ductus arteriosus: Implications for transcatheter therapy. Catheter. Cardiovasc. Interv. 2004, 61, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Clyman, R.I. Mechanisms Regulating the Ductus Arteriosus. Biol. Neonate 2006, 89, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Kajino, H.; Goldbarg, S.; Roman, C.; Liu, B.M.; Mauray, F.; Chen, Y.Q.; Takahashi, Y.; Koch, C.J.; I Clyman, R. Vasa Vasorum Hypoperfusion Is Responsible for Medial Hypoxia and Anatomic Remodeling in the Newborn Lamb Ductus Arteriosus. Pediatr. Res. 2002, 51, 228–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vali, P.; Lakshminrusimha, S.; Pelech, A.; Underwood, M.; Ing, F. Patent ductus arteriosus in preterm infants: Is early transcatheter closure a paradigm shift? J. Perinatol. 2019, 39, 1449–1461. [Google Scholar] [CrossRef] [PubMed]

| GA 1 (weeks) | BW 2 (gr) | PA 3 (days) | PW 4 (gr) | PDA Type and Size at PA end 5 on TTE (mm) | Devices Tried 6 | Reason to Abandon Procedure | Delay between Catheterization and Spontaneous Closure (days) | |
|---|---|---|---|---|---|---|---|---|
| 1 | 27 + 1 | 1010 | 37 | 1685 | F/3 | No device | Spasm Too small | 0 |
| 2 | 24 + 2 | 515 | 36 | 875 | F/2.7 | 4*2 | Coarctation | 1 |
| 3 | 26 | 915 | 29 | 1370 | F/3 | 5*2 4*2 | LPA stenosis | 6 |
| 4 | 24 + 3 | 640 | 24 | 780 | F/3 | 4*2 | Coarctation Spasm | 3 |
| 5 | 24 + 2 | 610 | 28 | 890 | F (short 6 mm) 3.5 | 5*2 | Coarctation | 5 |
| 6 | 25 + 1 | 960 | 17 | 1100 | F/3 | 4*2 | LPA and aorta stenosis | Small, not hemodynamically significant |
| 7 | 25 + 6 | 850 | 21 | 1000 | F/4 | 5*2 | Unstable device | 2 |
| Patient | GA 1 (weeks) | BW 2 (gr) | PA 3 (days) | PW 4 (gr) | PDA Type and Size at PA End 5 on TTE (mm) | Devices Tried 6 | Reason to Abandon Procedure | Delay between Catheterization and Surgery (days) |
|---|---|---|---|---|---|---|---|---|
| 1 | 26 + 2 | 1000 | 38 | 1400 | F/3.7 | 5*2 | Spasm LPA stenosis | 1 |
| 2 | 23 + 3 | 765 | 18 | 900 | F (but short)/4 | 5*2 | Unstable device | 4 |
| 3 | 25 + 6 | 855 | 37 | 1425 | A/4.4 | Too large on angiography | 0 | |
| 4 | 23 + 4 | 600 | 37 | 960 | F/3.2 | 5*2 4*2 | LPA stenosis | 3 |
| 5 | 27 | NA | 37 | 900 | A/4.5 | 5*2 | Too large | 6 |
| Year | Spasm of the PDA in the Catheterization Lab | Surgical Closure of the PDA | Delayed Spontaneous Closure of the PDA |
|---|---|---|---|
| 2017 | 0 | 1 | 1 |
| 2018 | 1 | 2 | 0 |
| 2019 | 1 | 1 | 2 |
| 2020 | 0 | 1 | 2 |
| 2021 | 0 | 0 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méot, M.; Haddad, R.N.; Patkai, J.; Abu Zahira, I.; Di Marzio, A.; Szezepanski, I.; Bajolle, F.; Kermorvant, E.; Lapillonne, A.; Bonnet, D.; et al. Spontaneous Closure of the Arterial Duct after Transcatheter Closure Attempt in Preterm Infants. Children 2021, 8, 1138. https://doi.org/10.3390/children8121138
Méot M, Haddad RN, Patkai J, Abu Zahira I, Di Marzio A, Szezepanski I, Bajolle F, Kermorvant E, Lapillonne A, Bonnet D, et al. Spontaneous Closure of the Arterial Duct after Transcatheter Closure Attempt in Preterm Infants. Children. 2021; 8(12):1138. https://doi.org/10.3390/children8121138
Chicago/Turabian StyleMéot, Mathilde, Raymond N. Haddad, Juliana Patkai, Ibrahim Abu Zahira, Anna Di Marzio, Isabelle Szezepanski, Fanny Bajolle, Elsa Kermorvant, Alexandre Lapillonne, Damien Bonnet, and et al. 2021. "Spontaneous Closure of the Arterial Duct after Transcatheter Closure Attempt in Preterm Infants" Children 8, no. 12: 1138. https://doi.org/10.3390/children8121138







