Clinical Significance of the Fetuin-A-to-Adiponectin Ratio in Obese Children and Adolescents with Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Definition of Obesity, DM, and MetS
2.3. Anthropometric and Laboratory Parameters
2.4. Fetuin-A and Adiponectin Measurements
2.5. Statistics
3. Results
3.1. Characteristics of Study Participants
3.2. Fetuin-A, Adiponectin, and FAR of Diabetic Children and Controls in Relation to BMI SDS
3.3. Association of Fetuin-A, Adiponectin, and FAR with Metabolic Parameters of Diabetic Children
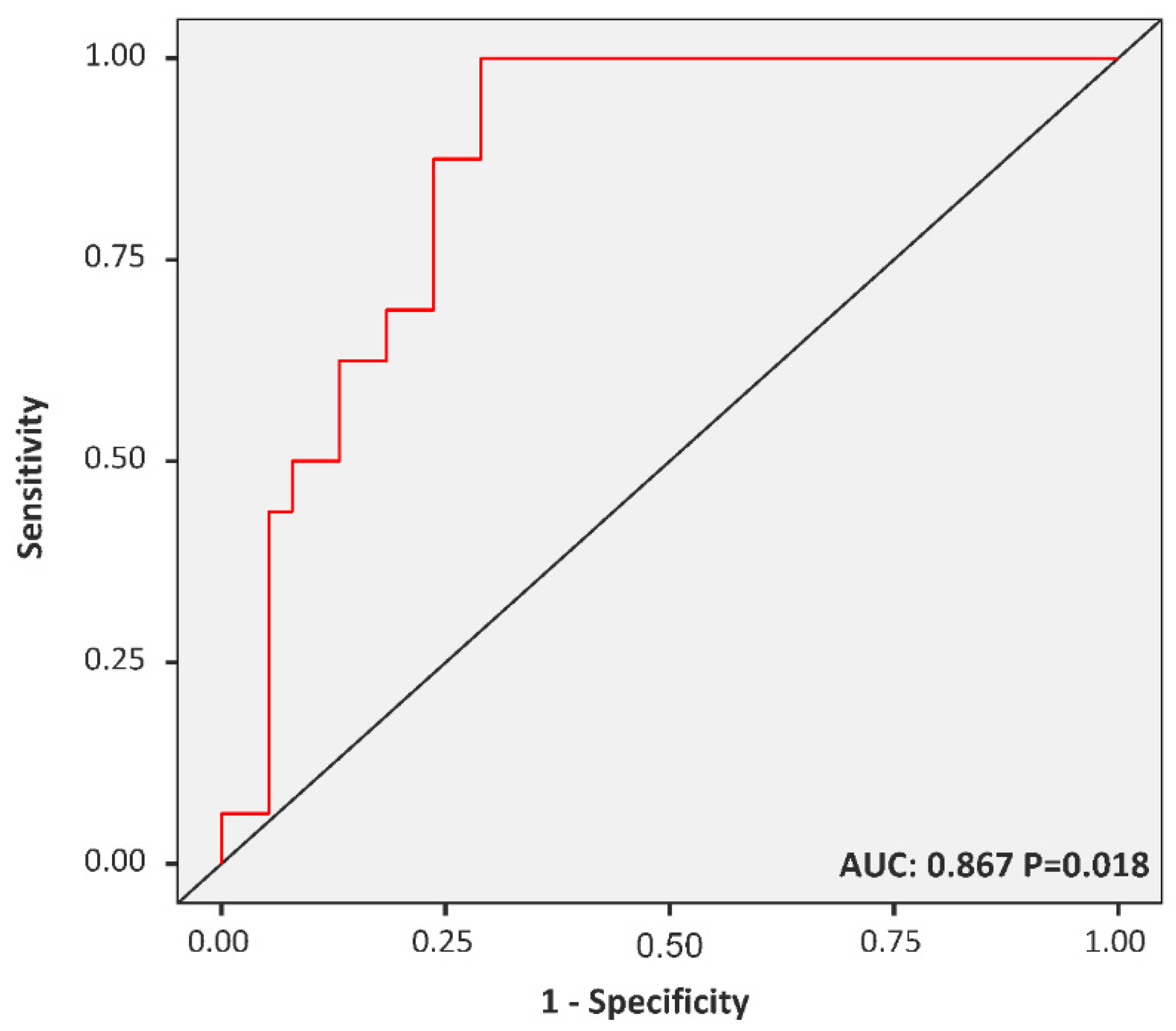
3.4. The FAR Cut-off Predicting MetS among Diabetic Children
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef]
- Kim, J.H.; Lim, J.S. Trends of Diabetes and Prediabetes Prevalence among Korean Adolescents from 2007 to 2018. J. Korean Med. Sci. 2021, 36, e112. [Google Scholar] [CrossRef]
- Koren, D.; Levitsky, L.L. Type 2 Diabetes Mellitus in Childhood and Adolescence. Pediatr. Rev. 2021, 42, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Sevaliev, N.; Strich, D.; Avnon-Ziv, C.; Levy-Khademi, F. The metabolic consequences of overweight in a cohort of children with type 1 diabetes. J. Pediatr. Endocrinol. Metab. 2019, 32, 715–719. [Google Scholar] [CrossRef]
- Corbin, K.D.; Driscoll, K.A.; Pratley, R.E.; Smith, S.R.; Maahs, D.M.; Mayer-Davis, E.J.; Advancing Care for Type, D.; Obesity, N. Obesity in Type 1 Diabetes: Pathophysiology, Clinical Impact, and Mechanisms. Endocr. Rev. 2018, 39, 629–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esser, N.; Utzschneider, K.M.; Kahn, S.E. Early beta cell dysfunction vs. insulin hypersecretion as the primary event in the pathogenesis of dysglycaemia. Diabetologia 2020, 63, 2007–2021. [Google Scholar] [CrossRef] [PubMed]
- Dogru, T.; Kirik, A.; Gurel, H.; Rizvi, A.A.; Rizzo, M.; Sonmez, A. The Evolving Role of Fetuin-A in Nonalcoholic Fatty Liver Disease: An Overview from Liver to the Heart. Int. J. Mol. Sci. 2021, 22, 6627. [Google Scholar] [CrossRef]
- Bayramoglu, E.; Cetinkaya, S.; Ozalkak, S.; Kurnaz, E.; Demirci, G.; Ozturk, H.S.; Savas-Erdeve, S.; Aycan, Z. Evaluation of the pathophysiological role of Fetuin A levels in adolescents with polycystic ovary syndrome. J. Pediatr. Endocrinol. Metab. 2021, 34, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Wang, S.; Yang, M.N.; Dong, Y.; He, H.; Fang, F.; Huang, R.; Yu, X.G.; Zhang, G.H.; Zhao, X.; et al. Fetuin-A in Infants Born Small- or Large-for-Gestational-Age. Front. Endocrinol. 2020, 11, 567955. [Google Scholar] [CrossRef]
- Reinehr, T. Inflammatory markers in children and adolescents with type 2 diabetes mellitus. Clin. Chim. Acta 2019, 496, 100–107. [Google Scholar] [CrossRef]
- Pena, A.S.; Harrington, J.; Peters Black, S.K.; Gent, R.; Hirte, C.; Couper, J.J.; Wiltshire, E. Vascular function and distribution of adiponectin isomers during puberty in children and adolescents with obesity. Horm. Res. Paediatr. 2021, 94, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Ramon-Krauel, M.; Leal-Witt, M.J.; Osorio-Conles, O.; Amat-Bou, M.; Lerin, C.; Selva, D.M. Relationship between adiponectin, TNFalpha, and SHBG in prepubertal children with obesity. Mol. Cell. Pediatr. 2021, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T.; Woelfle, J.; Wiegand, S.; Karges, B.; Meissner, T.; Nagl, K.; Holl, R.W. Leptin but not adiponectin is related to type 2 diabetes mellitus in obese adolescents. Pediatr. Diabetes 2016, 17, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Icer, M.A.; Yildiran, H. Effects of nutritional status on serum fetuin-A level. Crit. Rev. Food Sci. Nutr. 2020, 60, 1938–1946. [Google Scholar] [CrossRef]
- Hennige, A.M.; Staiger, H.; Wicke, C.; Machicao, F.; Fritsche, A.; Haring, H.U.; Stefan, N. Fetuin-A induces cytokine expression and suppresses adiponectin production. PLoS ONE 2008, 3, e1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, H.; Zhou, Z.; Sun, M.; Chen, H. Association of fetuin-A to adiponectin ratio with metabolic syndrome: A cross-sectional study. Endocrine 2017, 58, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Davis, E.J.; Kahkoska, A.R.; Jefferies, C.; Dabelea, D.; Balde, N.; Gong, C.X.; Aschner, P.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr. Diabetes 2018, 19 (Suppl. 27), 7–19. [Google Scholar] [CrossRef]
- Kim, J.H.; Yun, S.; Hwang, S.S.; Shim, J.O.; Chae, H.W.; Lee, Y.J.; Lee, J.H.; Kim, S.C.; Lim, D.; Yang, S.W.; et al. The 2017 Korean National Growth Charts for children and adolescents: Development, improvement, and prospects. Korean J. Pediatr. 2018, 61, 135–149. [Google Scholar] [CrossRef]
- Weghuber, D.; Mangge, H.; Hochbrugger, E.; Stulnig, T.M. Impact of age and metabolic syndrome on the adipokine profile in childhood and adult obesity. Exp. Clin. Endocrinol. Diabetes 2014, 122, 363–367. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Wigger, M.; Schaible, J.; Muscheites, J.; Kundt, G.; Haffner, D.; Fischer, D.C. Fetuin-A serum concentrations in healthy children. Ann. Clin. Biochem. 2009, 46, 511–513. [Google Scholar] [CrossRef]
- Lausten-Thomsen, U.; Lund, M.A.V.; Frithioff-Bojsoe, C.; Hedley, P.L.; Pedersen, O.; Hansen, T.; Christiansen, M.; Holm, J.C. Reference values for leptin/adiponectin ratio in healthy children and adolescents. Clin. Chim. Acta 2019, 493, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, J.P.; Quiroz, R.; Nelson, K.P.; Teng, Z.; Keaney, J.F., Jr.; Sullivan, L.M.; Vasan, R.S. Prospective Relation of Circulating Adipokines to Incident Metabolic Syndrome: The Framingham Heart Study. J. Am. Heart Assoc. 2017, 6, e004974. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, M.; Jin, H.; Chen, H.; Ju, H. Fetuin-a to adiponectin ratio is a sensitive indicator for evaluating metabolic syndrome in the elderly. Lipids Health Dis. 2020, 19, 61. [Google Scholar] [CrossRef] [Green Version]
- Wondmkun, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- Bourebaba, L.; Marycz, K. Pathophysiological Implication of Fetuin-A Glycoprotein in the Development of Metabolic Disorders: A Concise Review. J. Clin. Med. 2019, 8, 2033. [Google Scholar] [CrossRef] [Green Version]
- Yahia, S.; El-Farahaty, R.; El-Gilany, A.H.; Shoaib, R.; Ramadan, R.; Salem, N. Serum adiponectin, body adiposity and metabolic parameters in obese Egyptian children with Down syndrome. J. Pediatr. Endocrinol. Metab. 2021, 34, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Akcan, N.; Obaid, M.; Salem, J.; Bundak, R. Evidence in obese children: Contribution of tri-ponderal mass index or body mass index to dyslipidemia, obesity-inflammation, and insulin sensitivity. J. Pediatr. Endocrinol. Metab. 2020, 33, 223–231. [Google Scholar] [CrossRef]
- Gerst, F.; Wagner, R.; Kaiser, G.; Panse, M.; Heni, M.; Machann, J.; Bongers, M.N.; Sartorius, T.; Sipos, B.; Fend, F.; et al. Metabolic crosstalk between fatty pancreas and fatty liver: Effects on local inflammation and insulin secretion. Diabetologia 2017, 60, 2240–2251. [Google Scholar] [CrossRef]
- Pyziak-Skupien, A.; Bobeff, K.; Wyka, K.; Banach, K.; Malachowska, B.; Fendler, W.; Szadkowska, A.; Mlynarski, W.; Zmyslowska, A. Fetuin-A and Interleukine-8 in Children with the Clinical Remission of Type 1 Diabetes. Iran. J. Immunol. 2020, 17, 144–153. [Google Scholar]

| Clinical Parameters | Total (n = 98) | NOB (n = 45) | OB (n = 53) |
|---|---|---|---|
| Male, n (%) | 55 (58.1) | 21 (46.7) | 34 (64.2) |
| Age, years | 12.42 (9.56, 16.17) | 12.08 (9.42, 16.04) | 12.08 (9.92, 16.31) |
| Institution of enrollment | |||
| Seoul St. Mary’s Hospital, n (%) | 64 (65.3) | 28 (62.2) | 26 (49.1) |
| Bucheon St. Mary’s Hospital, n (%) | 34 (34.7) | 17 (37.8) | 27 (50.9) |
| Diabetes mellitus, n (%) | 54 (55.1) | 27 (50.9) | |
| Type 1, n (%) | 31 (57.4) | 7 (25.9) | |
| Type 2, n (%) | 23 (42.6) | 27 (60.0) | 20 (74.1) |
| Duration, years | 1.58 (1.00, 4.13) | 24 (88.9) | 1.67 (1.25, 3.83) |
| MetS, n (%) | 19 (19.4) | 3 (11.1) | 19 (35.8) |
| Controls, n (%) | 3 (15.8) | 1.5 (0.5, 4.5) | 4 (21.1) |
| Type 1, n (%) | 4 (21.1) | 0 (0.0) | 12(63.2) |
| Type 2, n (%) | 12 (63.2) | 3 (15.8) | |
| Anthropometry | |||
| Height SDS | 0.43 ± 1.14 | 0.00 ± 1.01 | 0.80 ± 1.09 |
| Weight SDS | 1.42 ± 1.75 | −0.17 ± 0.99 | 2.78 ± 0.86 |
| BMI SDS | 1.58 ± 1.95 | −0.19 ± 1.10 | 3.09 ± 0.99 |
| Total (n = 98) | p | ||||
|---|---|---|---|---|---|
| OC (n = 26) | NOC (n = 18) | ODM (n = 18) | NODM (n = 36) | ||
| Age (years) 2,3,4,5,6 | 10.54 (9.42, 12.04) | 8.92 (8.60, 10.48) | 15.83 (15.00, 18.17) | 14.67 (10.8, 16.58) | <0.001 |
| BMI SDS 1,3,4,6 | 2.73 (2.46, 3.24) | 0.24 (−0.56, 1.05) | 3.03 (2.43, 3.99) | −0.62 (−1.44, 0.44) | <0.001 |
| CRP (mg/dL) 1,2,4,5,6 | 0.10 (0.05, 0.19) | 0.03 (0.03, 0.10) | 0.87 (0.19, 2.60) | 0.30 (0.03, 0.48) | <0.001 |
| Glucose (mg/dL) 2,3,4,5,6 | 91 (91, 97.50) | 96 (90, 97.50) | 161 (113, 248) | 110 (100, 128) | <0.001 |
| AST (U/L) 3,5,6 | 22 (18, 28) | 24 (20, 28.50) | 27 (16, 59) | 17 (13, 19) | <0.001 |
| ALT (U/L) 1,3,4,6 | 18 (13, 41) | 14 (9, 16.50) | 54 (16, 103) | 10 (9, 14) | <0.001 |
| TC (mg/dL) | 170 (156.50, 196) | 179 (150.50, 222) | 182 (159, 205) | 170 (149, 199) | 0.770 |
| TG (mg/dL) 2,6 | 98 (56, 129.50) | 96 (75, 150.50) | 151 (109, 238) | 78 (46, 116) | 0.002 |
| HDL-C (mg/dL) 6 | 49 (44, 55.50) | 52 (44, 64.50) | 45 (40, 55) | 57 (50, 67) | 0.003 |
| LDL-C (mg/dL) | 104 (95.50, 128) | 108 (84.5, 131) | 108.80 (81.40, 124) | 96 (77.20, 127) | 0.401 |
| Uric acid (mg/dL) 1,3,4,6 | 6.35 (4.88, 7.13) | 4.30 (3.80, 4.88) | 5.40 (4.50, 6.30) | 4.40 (3.50, 5.30) | <0.001 |
| HbA1c (%) 1,2,3,4,5 | 5.50 (5.38, 5.70) | 5.20 (5.08, 5.43) | 8.80 (6.70, 10.30) | 7.60 (6.70, 9.10) | <0.001 |
| C-peptide (ng/mL) 1,3,5,6 | 2.63 (2.18, 3.79) | 2.01 (1.53, 2.75) | 2.56 (0.90, 4.21) | 0.17 (0.02, 0.69) | <0.001 |
| HOMA-IR 3,4,5,6 | 4.79 (2.73, 6.50) | 2.46 (1.77, 4.94) | 6.33 (3.51, 12.84) | 0.84 (0.38, 1.48) | <0.001 |
| HOMA-β 1,2,3,5,6 | 229.24 (171.40, 299.24) | 124.65 (89.18, 225.73) | 75.21 (26.54, 163.44) | 26.02 (9.75, 50.40) | <0.001 |
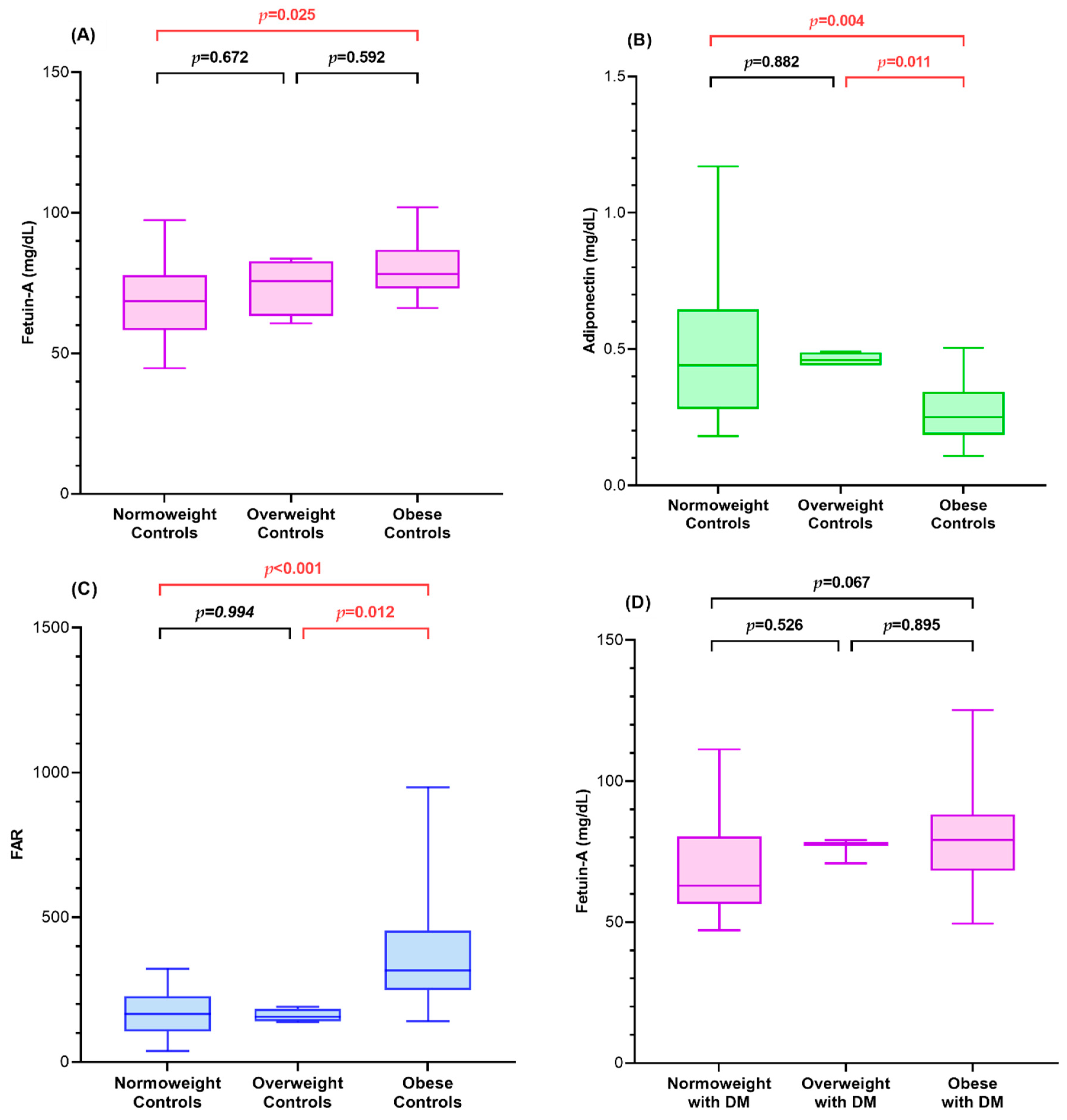
| Fetuin-A (mg/dL) 1,3 | 78.14 (73.06, 86.75) | 70.16 (60.18, 80.93) | 79.25 (68.27, 88.19) | 65.52 (57.09, 80.20) | 0.006 |
| Adiponectin (mg/dL) 1,3,4,6 | 0.25 (0.18, 0.34) | 0.45 (0.36, 0.52) | 0.23 (0.13, 0.35) | 0.56 (0.33, 0.91) | <0.001 |
| FAR 1,3,4,6 | 316.38 (248.98, 454.17) | 159.07 (123.38, 204.07) | 341.56 (232.41, 653.07) | 129.43 (60.79, 220.62) | <0.001 |
| Total (n = 98) | p | |||
|---|---|---|---|---|
| Controls (n = 44) | T1DM (n = 31) | T2DM (n = 23) | ||
| Age (years) 1,2,3 | 9.92 (8.67, 11.58) | 14.67 (10.08, 16.42) | 17.58 (15.75, 18.25) | <0.001 |
| BMI SDS 1,2,3 | 2.22 (0.78, 2.92) | 0.15 (−1.16, 1.59) | 3.19 (1.99, 4.66) | <0.001 |
| CRP (mg/dL) 1,3 | 0.07 (0.03, 0.14) | 0.3 (0.03, 0.73) | 0.87 (0.12, 2.60) | <0.001 |
| Glucose (mg/dL) 1,3 | 94 (90.75, 97.25) | 113 (101, 161) | 122 (112, 219) | <0.001 |
| AST (U/L) 1 | 22.5 (18.75, 28.25) | 17 (14, 21) | 34 (13, 59) | 0.002 |
| ALT (U/L) 1,2,3 | 15 (11.75, 22.5) | 12 (9, 15) | 57 (12, 103) | <0.001 |
| TC (mg/dL) | 173.5 (155.75, 203.75) | 170 (149, 204) | 181 (159, 202) | 0.981 |
| TG (mg/dL) 3 | 97 (58.75, 133.5) | 79 (50, 156) | 127 (99, 230) | 0.025 |
| HDL-C (mg/dL) 1,2,3 | 50 (44, 60.25) | 59 (52, 71) | 44 (39, 49) | <0.001 |
| LDL-C (mg/dL) | 106 (90.75, 127) | 90.4 (74, 124) | 116.6 (90, 123.2) | 0.118 |
| Uric acid (mg/dL) | 5.25 (4.3, 6.85) | 4.40 (3.8, 5.3) | 5.4 (4.7, 6.4) | 0.002 |
| HbA1c (%) 1,2 | 5.4 (5.2, 5.6) | 8.5 (6.8, 9.6) | 7.9 (6.5, 10.3) | <0.001 |
| C-peptide (ng/mL) 1,3 | 2.45 (1.83, 3.42) | 0.13 (0.02, 0.66) | 3.26 (1.96, 4.61) | <0.001 |
| HOMA-IR 1,2,3 | 3.87 (2.26, 5.72) | 0.95 (0.38, 1.82) | 6.59 (4.27, 12.84) | <0.001 |
| HOMA-β (%) 1,2,3 | 200.35 (136.19, 292.07) | 22.76 (9.53, 42.45) | 107.92 (60.04, 209.39) | <0.001 |
| Fetuin-A (mg/dL) | 75.59 (68.32, 83.62) | 66.24 (59.99, 80.41) | 79.25 (68.27, 84.53) | 0.128 |
| Adiponectin (mg/dL) 1,2,3 | 0.31 (0.21, 0.44) | 0.56 (0.33, 0.94) | 0.19 (0.13, 0.31) | <0.001 |
| FAR 1,2,3 | 252.48 (166.87, 346.68) | 129.43 (60.79, 245.54) | 407.77 (243.57, 653.07) | <0.001 |
| Diabetic Children (n = 54) | Controls (n = 44) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fetuin-A | Adiponectin | FAR | Fetuin-A | Adiponectin | FAR | |||||||
| ρ | p | Ρ | p | ρ | p | ρ | p | ρ | p | ρ | p | |
| BMI SDS | 0.404 | 0.002 | −0.649 | <0.001 | 0.679 | <0.001 | 0.437 | 0.003 | −0.455 | 0.002 | 0.540 | <0.001 |
| AST | 0.428 | 0.001 | −0.297 | 0.029 | 0.381 | 0.004 | −0.076 | 0.624 | −0.031 | 0.843 | −0.004 | 0.980 |
| ALT | 0.430 | 0.001 | −0.496 | <0.001 | 0.560 | <0.001 | 0.099 | 0.523 | −0.376 | 0.012 | 0.358 | 0.017 |
| TC | 0.121 | 0.385 | 0.031 | 0.826 | 0.008 | 0.953 | 0.083 | 0.592 | −0.185 | 0.230 | 0.143 | 0.354 |
| TG | 0.247 | 0.072 | −0.512 | <0.001 | 0.517 | <0.001 | 0.156 | 0.311 | −0.130 | 0.400 | 0.119 | 0.442 |
| HDL-C | −0.303 | 0.026 | 0.688 | <0.001 | −0.663 | <0.001 | −0.167 | 0.277 | 0.288 | 0.058 | −0.300 | 0.048 |
| LDL-C | 0.162 | 0.241 | −0.229 | 0.096 | 0.229 | 0.096 | 0.114 | 0.462 | −0.292 | 0.054 | 0.250 | 0.102 |
| Uric acid | 0.112 | 0.421 | −0.522 | <0.001 | 0.493 | <0.001 | 0.214 | 0.163 | −0.577 | <0.001 | 0.594 | <0.001 |
| HbA1c | 0.113 | 0.415 | 0.140 | 0.313 | −0.084 | 0.547 | 0.222 | 0.148 | −0.464 | 0.002 | 0.453 | 0.002 |
| C-peptide | 0.246 | 0.072 | −0.776 | <0.001 | 0.745 | <0.001 | 0.249 | 0.104 | −0.512 | <0.001 | 0.560 | <0.001 |
| HOMA-IR | 0.395 | 0.003 | −0.680 | <0.001 | 0.699 | <0.001 | 0.068 | 0.662 | −0.462 | 0.002 | 0.452 | 0.002 |
| HOMA-β | 0.200 | 0.147 | −0.581 | <0.001 | 0.560 | <0.001 | 0.045 | 0.772 | −0.399 | 0.007 | 0.390 | 0.009 |
| Risk Factors | Diabetic Children (n = 54) | Controls (n = 44) | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| β | p | β | p | β | p | Β | p | |
| Age | 15.9 | 0.364 | 3.74 | 0.819 | 41.7 | <0.001 | 31.7 | 0.002 |
| Sex | 173 | 0.126 | 180.74 | 0.080 | 95.3 | 0.098 | −24.3 | 0.635 |
| BMI SDS | 86.7 | <0.001 | 85.57 | <0.001 | 63.0 | <0.001 | 49.6 | 0.004 |
| AST | 5.69 | 0.027 | 3.39 | 0.004 | ||||
| ALT | 3.49 | 0.006 | 1.66 | 0.001 | ||||
| TC | −2.02 | 0.275 | 0.695 | 0.445 | ||||
| TG | 0.97 | 0.125 | 0.613 | 0.093 | ||||
| HDL-C | −12.50 | 0.002 | −4.75 | 0.065 | ||||
| LDL-C | −0.31 | 0.881 | 0.908 | 0.354 | ||||
| Uric acid | 160.00 | <0.001 | 65.3 | <0.001 | ||||
| HbA1c | −1.23 | 0.963 | 45.1 | 0.251 | ||||
| C-peptide | 115 | <0.001 | 54.7 | 0.011 | ||||
| HOMA-IR | 7.13 | 0.169 | 4.78 | 0.356 | ||||
| HOMA-β | 1.05 | 0.070 | 0.276 | 0.169 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, M.-B.; Kim, S.-K.; Kim, S.-H.; Cho, W.-K.; Suh, J.-S.; Cho, K.-S.; Suh, B.-K.; Jung, M.-H. Clinical Significance of the Fetuin-A-to-Adiponectin Ratio in Obese Children and Adolescents with Diabetes Mellitus. Children 2021, 8, 1155. https://doi.org/10.3390/children8121155
Ahn M-B, Kim S-K, Kim S-H, Cho W-K, Suh J-S, Cho K-S, Suh B-K, Jung M-H. Clinical Significance of the Fetuin-A-to-Adiponectin Ratio in Obese Children and Adolescents with Diabetes Mellitus. Children. 2021; 8(12):1155. https://doi.org/10.3390/children8121155
Chicago/Turabian StyleAhn, Moon-Bae, Seul-Ki Kim, Shin-Hee Kim, Won-Kyoung Cho, Jin-Soon Suh, Kyoung-Soon Cho, Byung-Kyu Suh, and Min-Ho Jung. 2021. "Clinical Significance of the Fetuin-A-to-Adiponectin Ratio in Obese Children and Adolescents with Diabetes Mellitus" Children 8, no. 12: 1155. https://doi.org/10.3390/children8121155
APA StyleAhn, M.-B., Kim, S.-K., Kim, S.-H., Cho, W.-K., Suh, J.-S., Cho, K.-S., Suh, B.-K., & Jung, M.-H. (2021). Clinical Significance of the Fetuin-A-to-Adiponectin Ratio in Obese Children and Adolescents with Diabetes Mellitus. Children, 8(12), 1155. https://doi.org/10.3390/children8121155






