Low- and High-Attenuation Lung Volume in Quantitative Chest CT in Children without Lung Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
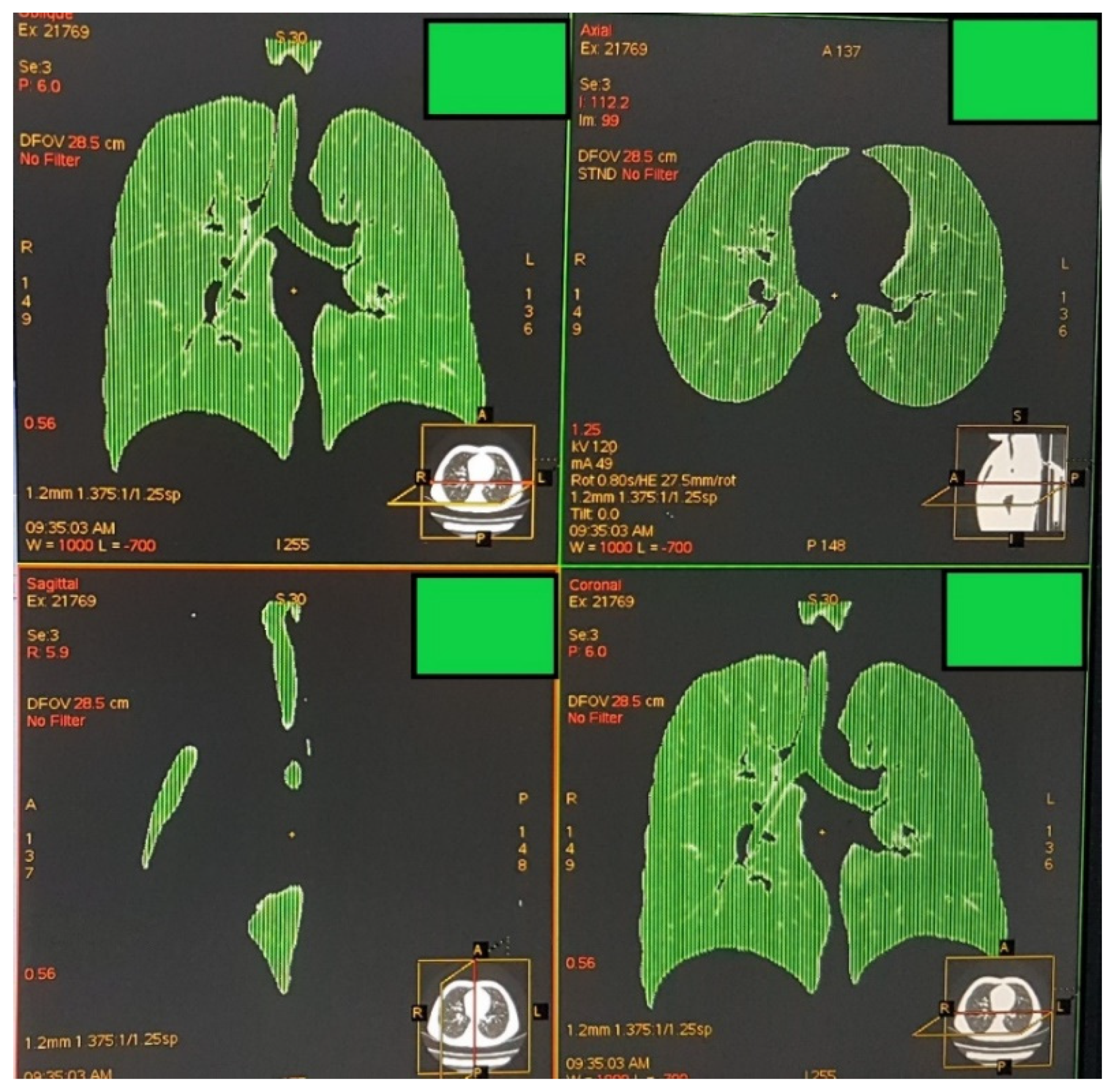
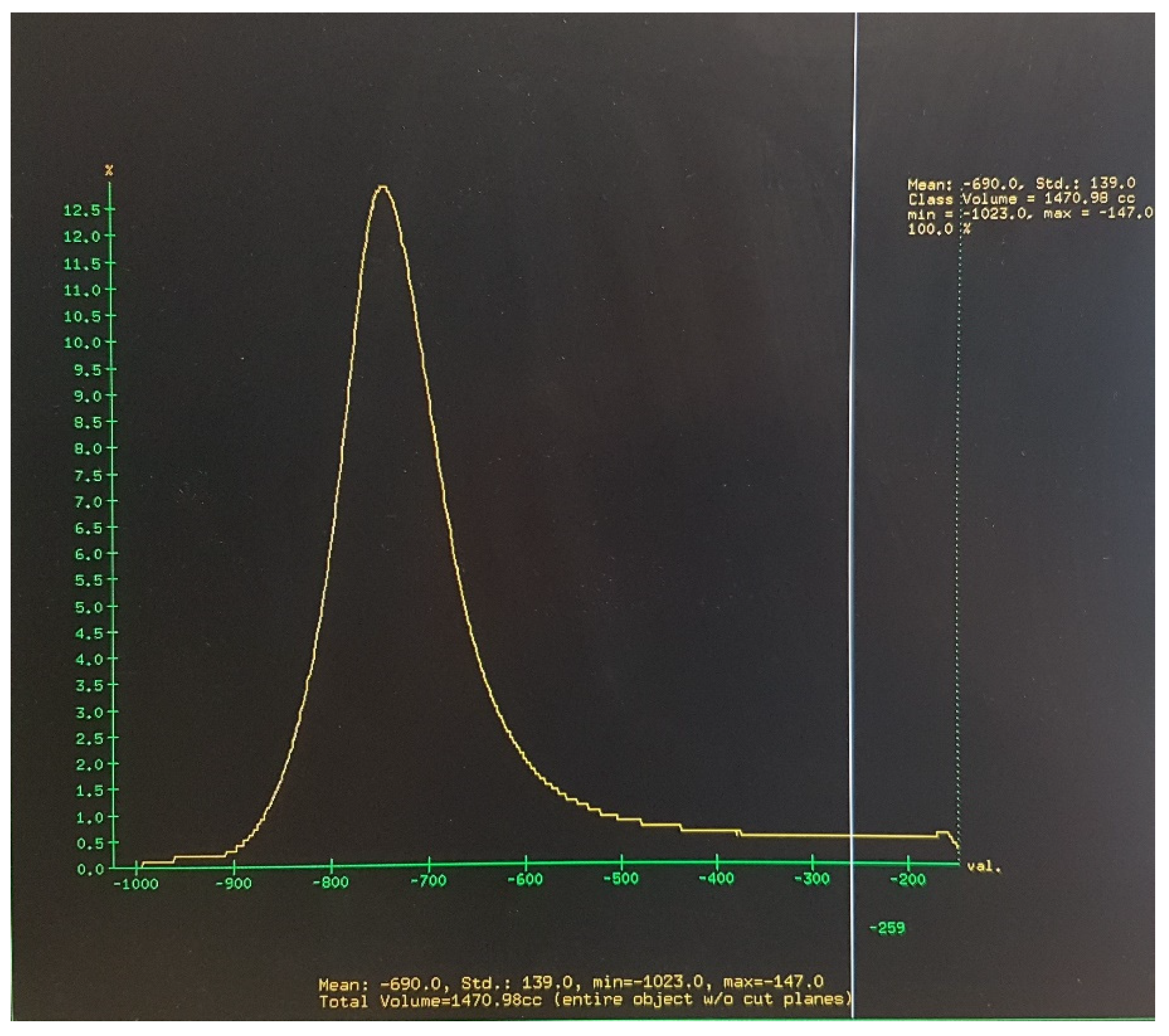
2.2. Image Acquisition, Analysis, and Definition of Attenuation Thresholds
2.3. Outcome Measures and Data Analysis
3. Results
3.1. Subjects’ Characteristics
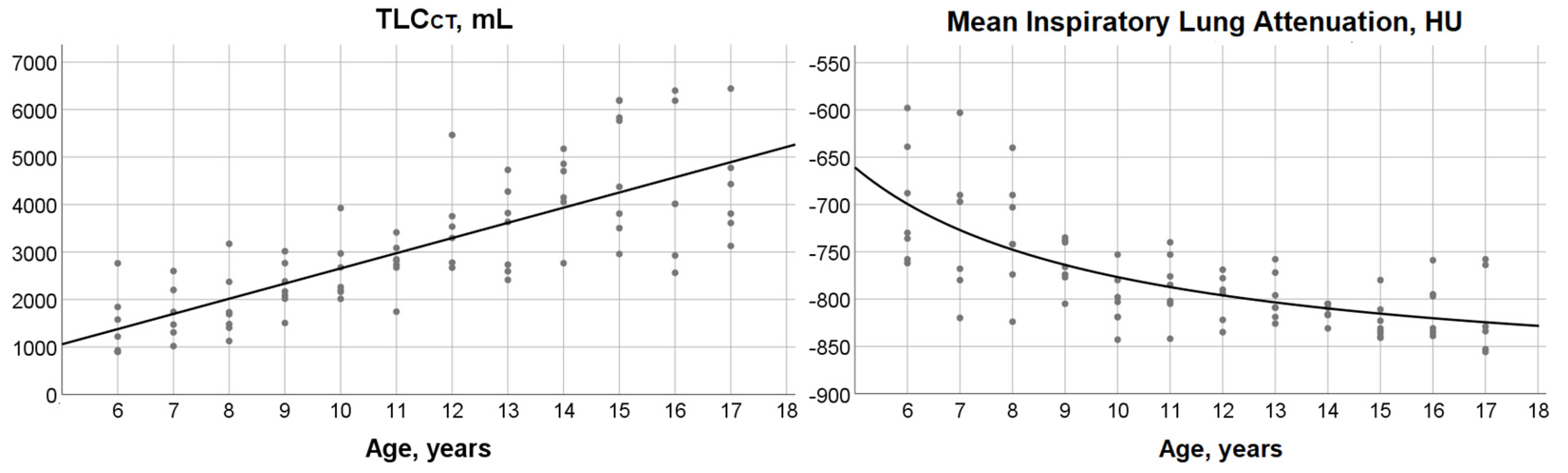
3.2. TLCCT, Mean Lung Attenuation at Full Inspiration and Their Relationship with Age
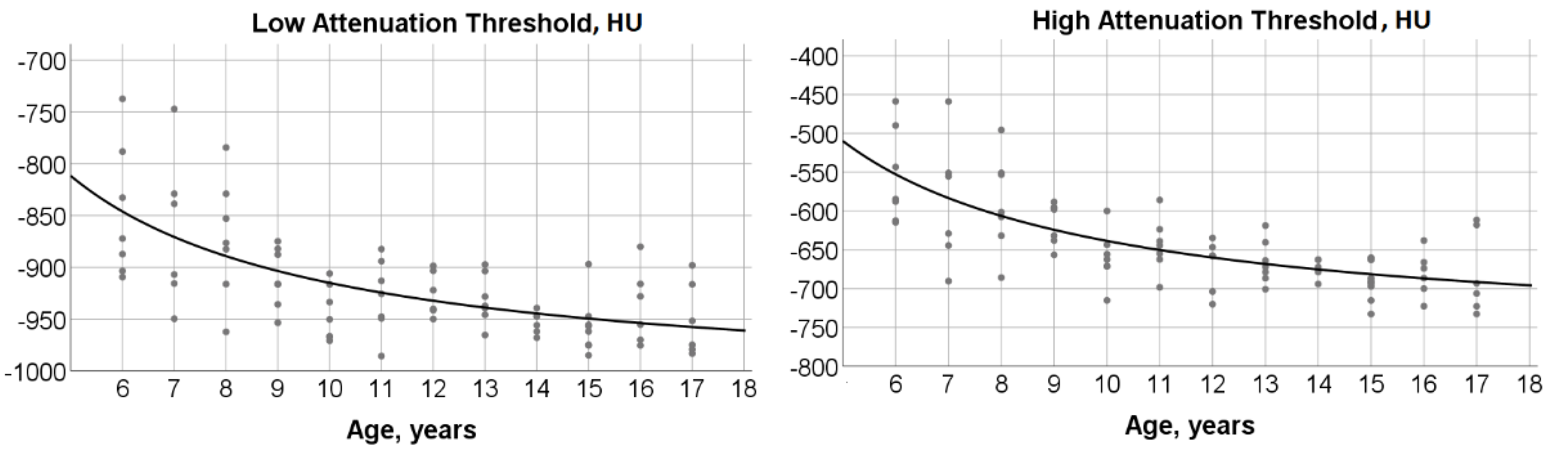
3.3. Age-Dependence of Low and High Attenuation Thresholds
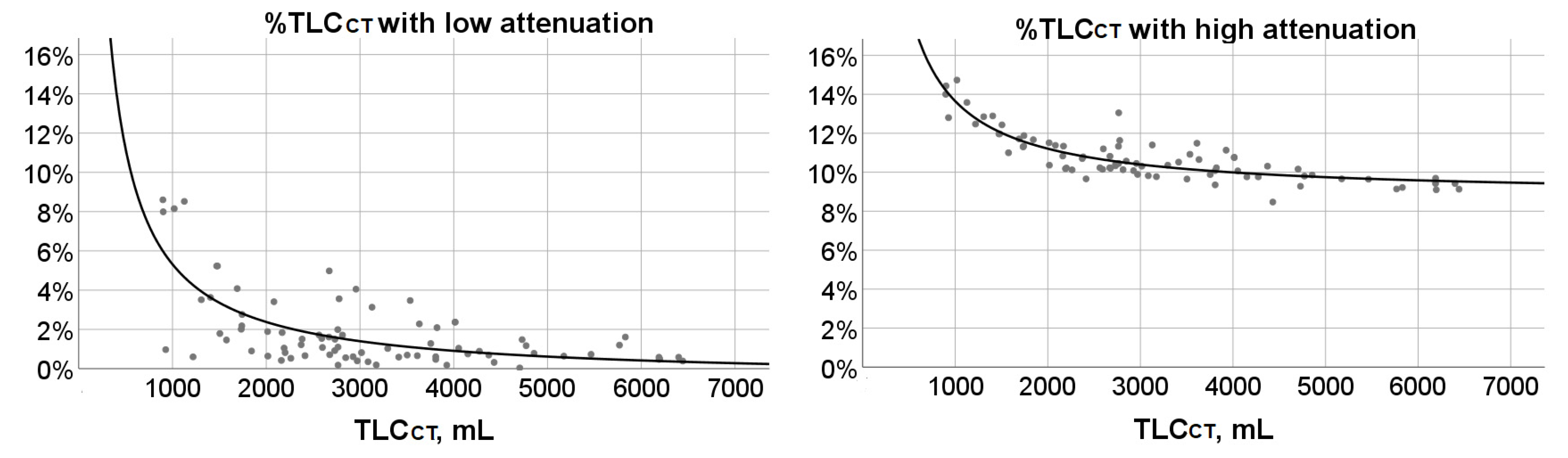
3.4. %TLCCT with Low or High Attenuation and Their Associations with Age and TLCCT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Litmanovich, D.; Boiselle, P.M.; Bankier, A.A. CT of pulmonary emphysema--current status, challenges, and future directions. Eur. Radiol. 2009, 19, 537–551. [Google Scholar] [CrossRef]
- Camiciottoli, G.; Cavigli, E.; Grassi, L.; Diciotti, S.; Orlandi, I.; Zappa, M.; Picozzi, G.; Pegna, A.L.; Paci, E.; Falaschi, F.; et al. Prevalence and correlates of pulmonary emphysema in smokers and former smokers. A densitometric study of participants in the ITALUNG trial. Eur. Radiol. 2009, 19, 58–66. [Google Scholar] [CrossRef]
- Marsh, S.; Aldington, S.; Williams, M.V.; Nowitz, M.R.; Kingzett-Taylor, A.; Weatherall, M.; Shirtcliffe, P.M.; McNaughton, A.A.; Pritchard, A.; Beasley, R. Utility of lung density measurements in the diagnosis of emphysema. Respir. Med. 2007, 101, 1512–1520. [Google Scholar] [CrossRef] [Green Version]
- Gevenois, P.A.; de Maertelaer, V.; De Vuyst, P.; Zanen, J.; Yernault, J.C. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am. J. Respir. Crit. Care Med. 1995, 152, 653–657. [Google Scholar] [CrossRef]
- Mohamed Hoesein, F.A.; van Rikxoort, E.; van Ginneken, B.; de Jong, P.A.; Prokop, M.; Lammers, J.W.; Zanen, P. Computed tomography-quantified emphysema distribution is associated with lung function decline. Eur. Respir. J. 2012, 40, 844–850. [Google Scholar] [CrossRef] [Green Version]
- Sarria, E.E.; Mattiello, R.; Rao, L.; Tiller, C.J.; Poindexter, B.; Applegate, K.E.; Granroth-Cook, J.; Denski, C.; Nguyen, J.; Yu, Z.; et al. Quantitative assessment of chronic lung disease of infancy using computed tomography. Eur. Respir. J. 2012, 39, 992–999. [Google Scholar] [CrossRef] [Green Version]
- Spielberg, D.R.; Walkup, L.L.; Stein, J.M.; Crotty, E.J.; Rattan, M.S.; Hossain, M.M.; Brody, A.S.; Woods, J.C. Quantitative CT scans of lung parenchymal pathology in premature infants ages 0–6 years. Pediatric. Pulmonol. 2018, 53, 316–323. [Google Scholar] [CrossRef]
- Szczesniak, R.; Turkovic, L.; Andrinopoulou, E.R.; Tiddens, H.A. Chest imaging in cystic fibrosis studies: What counts, and can be counted? J. Cyst. Fibros. 2017, 16, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Shin, H.J.; Sol, I.S.; Kim, S.Y.; Kim, J.D.; Yoon, H.; Kim, K.W.; Kim, M.J.; Lee, M.J.; Sohn, M.H. Lung Clearance Index and Quantitative Computed Tomography of Post-Infectious Bronchiolitis Obliterans in Infants. Sci. Rep. 2017, 7, 15128. [Google Scholar] [CrossRef] [Green Version]
- Higano, N.S.; Fleck, R.J.; Spielberg, D.R.; Walkup, L.L.; Hahn, A.D.; Thomen, R.P.; Merhar, S.L.; Kingma, P.S.; Tkach, J.A.; Fain, S.B.; et al. Quantification of neonatal lung parenchymal density via ultrashort echo time MRI with comparison to CT. J. Magn. Reson. Imaging 2017, 46, 992–1000. [Google Scholar] [CrossRef]
- Oudraad, M.C.J.; Kuo, W.; Rosenow, T.; Andrinopoulou, E.R.; Stick, S.M.; Tiddens, H. Assessment of early lung disease in young children with CF: A comparison between pressure-controlled and free-breathing chest computed tomography. Pediatr. Pulmonol. 2020, 55, 1161–1168. [Google Scholar] [CrossRef] [Green Version]
- Moutafidis, D.; Gavra, M.; Golfinopoulos, S.; Oikonomopoulou, C.; Kitra, V.; Woods, J.C.; Kaditis, A.G. Lung hyperinflation quantitated by chest CT in children with bronchiolitis obliterans syndrome following allogeneic hematopoietic cell transplantation. Clin. Imaging 2021, 75, 97–104. [Google Scholar] [CrossRef]
- Pennati, F.; Walkup, L.L.; Chhabra, A.; Towe, C.; Myers, K.; Aliverti, A.; Woods, J.C. Quantitative inspiratory-expiratory chest CT to evaluate pulmonary involvement in pediatric hematopoietic stem-cell transplantation patients. Pediatr. Pulmonol. 2021, 56, 1026–1035. [Google Scholar] [CrossRef]
- Barrera, C.A.; du Plessis, A.M.; Otero, H.J.; Mahtab, S.; Githinji, L.N.; Zar, H.J.; Zhu, X.; Andronikou, S. Quantitative CT analysis for bronchiolitis obliterans in perinatally HIV-infected adolescents-comparison with controls and lung function data. Eur. Radiol. 2020, 10, 4358–4368. [Google Scholar] [CrossRef]
- Kim, H.G.; Shin, H.J.; Kim, Y.H.; Sohn, M.H.; Lyu, C.J.; Kim, M.J.; Kim, K.W.; Lee, M.-J. Quantitative computed tomography assessment of graft-versus-host disease-related bronchiolitis obliterans in children: A pilot feasibility study. Eur. Radiol. 2015, 25, 2931–2936. [Google Scholar] [CrossRef]
- Cooper, B.G.; Stocks, J.; Hall, G.L.; Culver, B.; Steenbruggen, I.; Carter, K.W.; Thompson, B.R.; Graham, B.L.; Miller, M.R.; Ruppel, G.; et al. The Global Lung Function Initiative (GLI) Network: Bringing the world’s respiratory reference values together. Breathe 2017, 13, e56–e64. [Google Scholar] [CrossRef]
- Hall, G.L.; Filipow, N.; Ruppel, G.; Okitika, T.; Thompson, B.; Kirkby, J.; Steenbruggen, I.; Cooper, B.G.; Stanojevic, S. Official ERS technical standard: Global Lung Function Initiative reference values for static lung volumes in individuals of European ancestry. Eur. Respir. J. 2021, 57, 2000289. [Google Scholar] [CrossRef]
- Stein, J.M.; Walkup, L.L.; Brody, A.S.; Fleck, R.J.; Woods, J.C. Quantitative CT characterization of pediatric lung development using routine clinical imaging. Pediatr. Radiol. 2016, 46, 1804–1812. [Google Scholar] [CrossRef] [Green Version]
- Barrera, C.A.; Andronikou, S.; Tapia, I.E.; White, A.M.; Biko, D.M.; Rapp, J.B.; Zhu, X.; Otero, H.J. Normal age-related quantitative CT values in the pediatric lung: From the first breath to adulthood. Clin. Imaging 2021, 75, 111–118. [Google Scholar] [CrossRef]
- Cheng, T.; Li, Y.; Pang, S.; Wan, H.; Shi, G.; Cheng, Q.; Li, Q.; Pan, Z.; Huang, S. Normal lung attenuation distribution and lung volume on computed tomography in a Chinese population. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 1657–1668. [Google Scholar] [CrossRef] [Green Version]
- Mets, O.M.; van Hulst, R.A.; Jacobs, C.; van Ginneken, B.; de Jong, P.A. Normal range of emphysema and air trapping on CT in young men. AJR Am. J. Roentgenol. 2012, 199, 336–340. [Google Scholar] [CrossRef]
- Uhlving, H.H.; Andersen, C.B.; Christensen, I.J.; Gormsen, M.; Pedersen, K.D.; Buchvald, F.; Heilmann, C.; Nielsen, K.G.; Mortensen, J.; Moser, C.; et al. Biopsy-verified bronchiolitis obliterans and other noninfectious lung pathologies after allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2015, 21, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Faraci, M.; Ricci, E.; Bagnasco, F.; Pierri, F.; Giardino, S.; Girosi, D.; Olcese, R.; Castagnola, E.; Magnano, G.M.; Lanino, E. Imatinib melylate as second-line treatment of bronchiolitis obliterans after allogenic hematopoietic stem cell transplantation in children. Pediatr. Pulmonol. 2020, 55, 631–637. [Google Scholar] [CrossRef]
- Podolanczuk, A.J.; Oelsner, E.C.; Barr, R.G.; Hoffman, E.A.; Armstrong, H.F.; Austin, J.H.; Basner, R.C.; Bartels, M.N.; Christie, J.D.; Enright, P.L.; et al. High attenuation areas on chest computed tomography in community-dwelling adults: The MESA study. Eur. Respir. J. 2016, 48, 1442–1452. [Google Scholar] [CrossRef] [Green Version]
- Jagasia, M.H.; Greinix, H.T.; Arora, M.; Williams, K.M.; Wolff, D.; Cowen, E.W.; Tkaczyk, E.; Farhadfar, N.; Jain, S.; Steven, P.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol. Blood Marrow Transplant. 2015, 21, 389–401.e1. [Google Scholar] [CrossRef] [Green Version]
- L’Excellent, S.; Yakouben, K.; Delclaux, C.; Dalle, J.H.; Houdouin, V. Lung evaluation in 10-year survivors of pediatric allogeneic hematopoietic stem cell transplantation. Eur. J. Pediatr. 2019, 178, 1833–1839. [Google Scholar] [CrossRef]
- Gazourian, L.; Spring, L.; Meserve, E.; Hwang, D.; Diaz, A.A.; Ash, S.Y.; Ho, V.T.; Sholl, L.M.; Washko, G.R. Pulmonary Clinicopathological Correlation after Allogeneic Hematopoietic Stem Cell Transplantation: An Autopsy Series. Biol. Blood Marrow Transplant. 2017, 23, 1767–1772. [Google Scholar] [CrossRef] [Green Version]

| Age Group | Mean Lung Attenuation at Full Inspiration, HU | Low Attenuation Threshold, HU | High Attenuation Threshold, HU |
|---|---|---|---|
| 6-year-old (n = 7) | −730 (−758, −639) (−762, −598) | −872.3 (−903.5, −788.2) (−909.4, −737.3) | −584.7 (−612.5, −489.8) (−614.6, −458.7) |
| 7-year-old (n = 6) | −732.5 (−790, −668.3) (−820, −603) | −872.9 (−924.1, −808.5) (−949.7, −747.1) | −592.2 (−655.9, −528) (−690.3, −458.9) |
| 8-year-old (n = 7) | −742 (−774, −690) (−824, −640) | −876.5 (−916.2, −829) (−962.3, −784.3) | −601.5 (−916.2, −829) (−685.7, −495.7) |
| 9-year-old (n = 7) | −766 (−777, −738) (−805, −735) | −916.1 (−935.8, −882.1) (−953.5, −874.9) | −597.9 (−637.9, −595.1) (−656.5, −588.3) |
| 10-year-old (n = 7) | −803 (−819, −780) (−843, −753) | −950.3 (−967.1, −916.6) (−970.9, −906) | −662.4 (−671.3, −643.4) (−715.1, −600) |
| 11-year-old (n = 7) | −785 (−805, −753) (−842, −740) | −925.7 (−949.4, −894.1) (−985.7, −882.4) | −644.3 (−662.3, −623.6) (−698.3, −585.9) |
| 12-year-old (n = 6) | −792 (−825.3, −775.8) (−835, −769) | −931.2 (−943.6, −902.1) (−950, −898.6) | −657.7 (−707.8, −643.6) (−720, −634.7) |
| 13-year-old (n = 7) | −809 (−819, −772) (−826, −758) | −937.3 (−945.9, −903.6) (−965.4, −897.3) | −672.1 (−686.6, −640.4) (−700.7, −618.7) |
| 14-year-old (n = 6) | −812.5 (−820.5, −805) (−831, −805) | −951.7 (−963.5, −945.3) (−968, −939) | −674.1 (−682.5, −662.7) (−694, −662.7) |
| 15-year-old (n = 8) | −832.5 (−839, −814) (−841, −780) | −959.4 (−975.4, −949.4) (−985, −897) | −691.8 (−710.7, −668.9) (−732.7, −660.1) |
| 16-year-old (n = 6) | −814 (−836, −786) (−839, −759) | −941.8 (−971.5, −907.1) (−975.5, −880.1) | −680.2 (−705.6, −658.9) (−722.6, −637.9) |
| 17-year-old (n = 6) | −831.5 (−853.8, −762.5) (−856, −758) | −963.4 (−980.4, −911.9) (−983.3, −898.1) | −699.7 (−725.2, −616.3) (−732.6, −611.5) |
| Age Group | %TLCCT with Attenuation < Low Attenuation Threshold | %TLCCT with Attenuation > High Attenuation Threshold | TLCCT, mL | %TLCCT with Attenuation < Low Attenuation Threshold | %TLCCT with Attenuation > High Attenuation Threshold |
|---|---|---|---|---|---|
| 6-year-old (n = 7) | 1.46 (0.90, 7.99) (0.60, 8.60) | 12.47 (11.33, 14) (11, 14.43) | 897.3–1500 (n = 10) | 5.23 (2.88, 8.24) (0.60, 8.60) | 12.87 (12.35, 14.11) (11.97, 14.73) |
| 7-year-old (n = 6) | 2.85 (1.02, 5.96) (0.82, 8.15) | 11.66 (10.96, 13.32) (10.22, 14.73) | 1501–2000 (n = 7) | 2.01 (1.46, 2.76) (0.90, 4.08) | 11.67 (11.30, 11.88) (11.00, 12.43) |
| 8-year-old (n = 7) | 3.63 (1.22, 5.23) (0.19, 8.52) | 11.71 (10.70, 12.89) (9.77, 13.58) | 2001–2500 (n = 11) | 1.05 (0.64, 1.84) (0.42, 3.41) | 10.70 (10.18, 11.34) (9.66, 11.51) |
| 9-year-old (n = 7) | 1.51 (0.64, 1.84) (0.18, 3.41) | 11.34 (10.36, 12.43) (10.31, 13.05) | 2501–3000 (n = 17) | 1.50 (0.66, 1.86) (0.18, 4.98) | 10.43 (10.18, 11.01) (9.89, 13.05) |
| 10-year-old (n = 7) | 0.53 (0.40, 1.05) (0.18, 1.89) | 10.20 (10.12, 11.13) (9.89, 11.51) | 3001–3500 (n = 6) | 0.71(0.31, 1.56) (0.19, 3.13) | 10.34 (9.81, 10.74) (9.77, 11.40) |
| 11-year-old (n = 7) | 0.92 (0.56, 2.76) (0.35, 4.98) | 10.52 (10.13, 10.82) (9.82, 11.88) | 3501–4000 (n = 9) | 0.69 (0.54, 2.19) (0.18, 3.47) | 10.23 (9.77, 11.03) (9.35, 11.49) |
| 12-year-old (n = 6) | 1.45 (0.96, 3.49) (0.73, 3.56) | 10.30 (9.82, 11.10) (9.64, 11.63) | 4001–4500 (n = 7) | 0.89 (0.69, 2.37) (0.32, 2.37) | 10.07 (9.76, 10.76) (8.47, 10.76) |
| 13-year-old (n = 7) | 1.5 (0.89, 2.09) (0.66, 2.28) | 10.15 (9.66, 10.43) (9.28, 10.65) | 4501–5000 (n = 4) | 0.98 (0.23, 1.40) (0.04, 1.48) | 9.83 (9.41, 10.09) (9.28, 10.16) |
| 14-year-old (n = 6) | 0.77 (0.49, 1.06) (0.04, 1.10) | 9.97 (9.74, 10.23) (9.66, 10.45) | 5001–5500 (n = 2) | 0.69 (0.64, 0.73) | 9.65 (9.64, 9.66) |
| 15-year-old (n = 8) | 0.69 (0.48, 1.51) (0.47, 4.10) | 9.50 (9.16, 10.16) (9.10, 10.45) | 5501–6000 (n = 2) | 1.41 (1.20, 1.61) | 9.18 (9.14, 9.22) |
| 16-year-old (n = 6) | 1.17 (0.58, 2.37) (0.58, 2.37) | 10.23 (9.42, 10.76) (9.42, 10.76) | 6001–6500 (n = 5) | 0.50 (0.43, 0.58) (0.39, 0.58) | 9.42 (9.12, 9.56) (9.10, 9.69) |
| 17-year-old (n = 6) | 0.64 (0.37, 1.66) (0.32, 3.13) | 9.94 (8.97, 11.42) (8.47, 11.49) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moutafidis, D.; Gavra, M.; Golfinopoulos, S.; Kattamis, A.; Chrousos, G.; Kanaka-Gantenbein, C.; Kaditis, A.G. Low- and High-Attenuation Lung Volume in Quantitative Chest CT in Children without Lung Disease. Children 2021, 8, 1172. https://doi.org/10.3390/children8121172
Moutafidis D, Gavra M, Golfinopoulos S, Kattamis A, Chrousos G, Kanaka-Gantenbein C, Kaditis AG. Low- and High-Attenuation Lung Volume in Quantitative Chest CT in Children without Lung Disease. Children. 2021; 8(12):1172. https://doi.org/10.3390/children8121172
Chicago/Turabian StyleMoutafidis, Dimitrios, Maria Gavra, Sotirios Golfinopoulos, Antonios Kattamis, George Chrousos, Christina Kanaka-Gantenbein, and Athanasios G. Kaditis. 2021. "Low- and High-Attenuation Lung Volume in Quantitative Chest CT in Children without Lung Disease" Children 8, no. 12: 1172. https://doi.org/10.3390/children8121172






