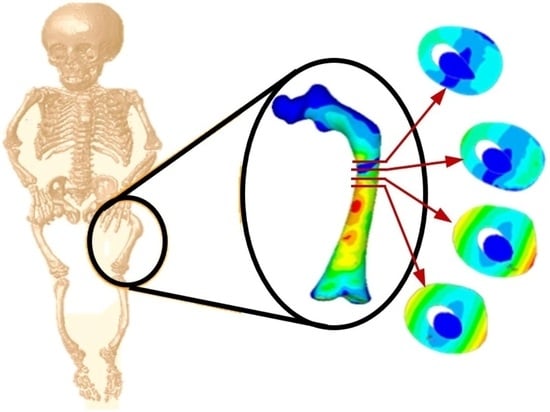
Bone Fractures Numerical Analysis in a Femur Affected by Osteogenesis Imperfecta
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methodology of 3D Modelling of an Infant’s Femur with OI Type III
2.2. Selection of the Minimum Unit of a 3D Image (Voxel)
2.3. Hounsfield Unit (HU) Assessment and Apparent Density (ρ) Evaluations for OI Bone Type III
2.4. Apparent Density (ρ) Evaluations for OI Bone Type III
2.5. Numerical 3D Model Analysis of OI Femur
3. Results
3.1. Transverse Fracture Study
3.2. Oblique Fracture Study
3.3. Comminuted Fracture Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harrington, J.; Sochett, E.; Howard, A. Update on the Evaluation and Treatment of Osteogenesis Imperfecta. Pediatr. Clin. N. Am. 2014, 61, 1243–1257. [Google Scholar] [CrossRef]
- Primorac, D.; Anticević, D.; Barisic, I. Osteogenesis imperfecta—Multi-systemic and life-long disease that affects whole family. Coll. Antropol. 2014, 38, 767–772. [Google Scholar]
- Binh, H.D.; Maasalu, K.; Dung, V.C. Las características clínicas de la osteogénesis imperfecta en Vietnam. Ortop. Int. (SICOT) 2017, 41, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Lindahl, K.; Langdahl, B.; Ljunggren, O. Treatment of osteogenesis imperfecta in adults. Eur. Soc. Endocrinol. 2014, 171, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munns, C.; Rauch, F.; Zeitlin, L. Delayed Osteotomy but Not Fracture Healing in Pediatric Osteogenesis Imperfecta Patients Receiving Pamidronate. J. Bone Miner. Res. 2004, 19, 1779–1986. [Google Scholar] [CrossRef] [Green Version]
- Anam, E.A.; Rauch, F.; Glorieux, F.H. Osteotomy Healing in Children with Osteogenesis Imperfecta Receiving Bisphosphonate Treatment. J. Bone Miner. Res. 2015, 30, 1362–1368. [Google Scholar] [CrossRef]
- Chotigavanichaya, C.; Jadhav, A.; Bernstein, R. Rod Diameter Prediction in Patients with Osteogenesis Imperfecta Undergoing Primary Osteotomy. J. Pediatr. Orthop. 2001, 21, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Azzam, K.; Rush, E.; Burke, B.; Nabower, A. Mid-term Results of Femoral and Tibial Osteotomies and Fassier-Duval Nailing in Children With Osteogenesis Imperfecta. J. Pediatr. Orthop. 2018, 38, 331–336. [Google Scholar] [CrossRef]
- Gimeno, S.; Pérez, C.; Guardiola, S. Epidemiología de la osteogénesis imperfecta: Una enfermedad rara en la Comunidad Valenciana. Rev. Esp. Salud Pública 2017, 91, e201711045. [Google Scholar]
- Alguacil, I.; Molina-Rueda, F.; Gómez, M. Tratamiento ortésico en pacientes con osteogénesis imperfecta. An. Pediatr. 2011, 74, 131.e1–131.e6. [Google Scholar] [CrossRef] [PubMed]
- Métaizeau, J.D. Fracturas diafisarias del fémur en el niño. EMC-Apar. Locomot. 2015, 48, 1–11. [Google Scholar] [CrossRef]
- González, P.; Rodríguez, M.; Castro, M. Fracturas diafisarias del fémur en el niño: Actualización en el tratamiento. Rev. Esp. Cir. Ortop. Traumatol. 2011, 55, 54–66. [Google Scholar] [CrossRef]
- Bubbear, J. Atypical Femur Fractures in Patients Treated with Biphosphonates: Identification, Management, and Prevention. Rambam Maimonides Med. J. 2016, 7, e0032. [Google Scholar] [CrossRef] [Green Version]
- Shane, E.; Burr, D.; Ebeling, P. Atipical subtrochanteric and diaphyseal femoral fractures: Report of a task force of the American Society for Bone and Mineral Research. J. Bone Min. Res. 2010, 25, 2267–2294. [Google Scholar] [CrossRef]
- Guiusti, A.; Hamdy, N.; Papapoulos, S. Atypical fractures of the femur and bisphosphonate therapy. A systematic review of case/case series studies. Bone 2010, 47, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Varga, P.; Baumbach, S.; Pahr, D. Validación de un modelo anatómico de elementos finitos específico de la fractura de Colles. J. Biomech. 2009, 42, 1726–1731. [Google Scholar] [CrossRef]
- Varga, P.; Willie, B.; Stephan, C. Finite element analysis of bone strength in osteogenesis imperfecta. Bone 2020, 133, 115250. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, L.A.; Sánchez, J.I.; Flores, J.A.; Torres-San Miguel, C.R. Numerical and Experimental Assessment of a Novel Anchored for Intramedullary Telescopic Nails Used in Osteogenesis Imperfecta Fractures. Appl. Sci. 2021, 11, 5422. [Google Scholar] [CrossRef]
- Li, X.; Vicenconti, M.; Cohen, M.C. Developing CT based computational models of pediatric femurs. J. Biomech. 2015, 48, 2034–2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helgason, B.; Perilli, E.; Schileo, E. Mathematical relationships between bone density and mechanical properties: A literature review. Clin. Biomech. 2008, 23, 135–146. [Google Scholar] [CrossRef]
- Manhard, M.; Nyman, J.; Does, M. Advances in imaging approaches to fracture risk evaluation. J. Lab. Clin. Med. 2017, 181, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Caouette, C.; Ikin, N.; Villemure, I. Geometry reconstruction method for patient-specific finite element models for the assessment of tibia fracture risk in osteogenesis imperfecta. Med. Biol. Eng. Comput. 2017, 55, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Altai, Z.; Vicencoti, M.; Offiah, A. Investigating the mechanical response of paediatric bone under bending and torsion using finite element analysis. Biomech. Model. Mechanobiol. 2018, 17, 1001–1009. [Google Scholar] [CrossRef] [Green Version]
- Fritz, J.; Grosland, N.; Smith, P. Finite Element Modeling and Analysis Applications in Osteogenesis Imperfecta. In Handbook of Transitional Care in Osteogenesis Imperfecta: Advances in Biology, Technology, and Clinical Practice, 1st ed.; Smith, P., Rauch, F., Harris, J., Eds.; Shriners Hospitals for Children Chicago: Chicago, IL, USA, 2016; pp. 149–160. [Google Scholar]
- Fritz, J.; Guan, Y.; Wang, M. A fracture risk assessment model of the femur in children with osteogenesis imperfecta (OI) during gait. J. Med. Eng. Phys. 2009, 31, 1043–1048. [Google Scholar] [CrossRef]
- Grassi, L.; Väänänen, S.; Ristinmaa, M. Prediction of femoral strength using 3D finite element models reconstructed from DXA images: Validation against experiments. Biomech. Model. Mechanobiol. 2017, 16, 989–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheong, V.; Masouros, S.; Bull, A. Fracture Simulation of Femoral Bone using Finite Element Method. In Proceedings of the IRCOBI Conference, Gothenburg, Sweden, 11–13 September 2013. IRC-13-96. [Google Scholar]
- Wanna, S.B.; Basaruddin, K.; Mat Som, M.H. Prediction on fracture risk of femur with Osteogenesis Imperfecta using finite element models: Preliminary study. J. Phys. Conf. Ser. 2017, 908, 012022. [Google Scholar] [CrossRef]
- Wanna, S.B.; Basaruddin, K.; Mat Som, M.H. Fracture risk prediction on children with Osteogenesis Imperfecta subjected to loads under activity of daily living. IOP Conf. Ser. Mater. Sci. Eng. 2018, 429, 012004. [Google Scholar] [CrossRef]
- Wanna, S.B.; Basaruddin, K.; Mat Som, M.H. Effect of loading direction on fracture of bone with osteogénesis imperfect (OI) during standing. AIP Conf. Proc. 2018, 2030, 020094. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Mat Som, M.H.; Basaruddin, K.S. Determination of Fracture Risk on Patient-specific Model of Femur with Osteogenesis Imperfecta. J. Phys. Conf. Ser. 2019, 1372, 012042. [Google Scholar] [CrossRef]
- Tan, L.C.; Mat Som, M.H.; Basaruddin, K.S. Biomechanical analysis of patient-specific femur model of osteogenesis imperfecta with cortical and cancellous bone. IOP Conf. Ser. Mater. Sci. Eng. 2019, 670, 012045. [Google Scholar] [CrossRef]
- Voon, T.T.; Mat Som, M.H.; Yazid, H. Segmentation of Cortical and Cancellous Bone with Osteogenesis Imperfecta using Thresholding-based Method. J. Phys. Conf. Ser. 2019, 1372, 012006. [Google Scholar] [CrossRef] [Green Version]
- Martínez, L.; García, A.; Espantaleón, M.; Monclús, J. Research to Increase Passive Safety of Affected by Osteogenesis Imperfecta. In Proceedings of the 13th International Conference, Protection of Children in Cars, Munich, Germany, 3–4 December 2015; pp. 1–22. [Google Scholar]
- Rueda, J.L.; Torres, C.R.; Ramírez, V.; Martínez, L. Design and Comparative Numerical Analysis of Designs of Intramedular Telescopic Systems for the Rehabilitation of Patients with Osteogenesis Imperfecta (OI) Type III. In Engineering Design Applications II Structures, Materials and Processes: Structures, Materials and Processes; Öchsner, A., Altenbach, H., Eds.; Springer: Cham, Switzerland, 2020; Volume 113, pp. 333–341. [Google Scholar] [CrossRef]
- Martínez, L.; Reed, M.P.; García, A. Crash Impact Dummies adapted to People Affected by Osteogenesis Imperfecta. In Proceedings of the IRCOBI Conference, Malaga, Spain, 14–16 September 2016. RC-16-97. [Google Scholar]
- Ramírez, V.; Torres, C.R.; Rueda, J.L. Finite Element Analysis of 3D Models of Upper and Lower Limbs of Mexican Patients with Osteogenesis Imperfecta (OI) Type III. In Engineering Design Applications II. Advanced Structured Materials; Öchsner, A., Altenbach, H., Eds.; Springer: Cham, Switzerland, 2020; Volume 113, pp. 343–353. [Google Scholar] [CrossRef]
- Rueda, J.L.; Torres, C.R.; Ramírez, V. Simulation by finite element method of intramedullary telescopic systems for rehabilitation of patients with osteogenesis imperfecta. Rev. Mex. Ing. Bioméd. 2019, 40, e201826. [Google Scholar] [CrossRef]
- Dellan, A.; Villarroel, M.; Hernández, A. Application of Hounsfield units in computed tomography as a diagnostic tool for intra-osseous lesions of the maxilla-mandibular complex: Diagnostic clinical study. Rev. Odontol. Univ. Cid. Sao Paulo 2015, 27, 100–111. [Google Scholar]
- Buroni, F.C.; Commisso, P.E.; Cisilino, A.P.; Sammartino, M. Determinación De Las Constantes Elásticas Anisótropas Del Tejido Óseo Utilizando Tomografías Computadas. Aplicación a La Construcción De Modelos De Elementos Finitos. Mec. Comput. 2004, XXIII. [Google Scholar]
- Taylor, W.R.; Roland, E.; Ploeg, H.; Hertig, D.; Klabunde, R.; Warner, M.D.; Hobatho, M.C.; Rakotomanana, L.; Clift, S.E. Determination of orthotropic bone elastic constants using FEA and modal analysis. J. Biomech. 2002, 35, 767–773. [Google Scholar] [CrossRef]
- Wirtz, D.C.; Schiffers, N.; Pandorf, T.; Radermacher, K.; Weichert, D.; Forst, R. Critical evaluation of known bone material properties to realize anisotropic FE-simulation of the proximal femur. J. Biomech. 2000, 33, 1325–1330. [Google Scholar] [CrossRef]
- Houcke, J.; Khanduja, V.; Pattyn, C. The History of Biomechanics in Total Hip Arthroplasty. Indian J. Orthop. 2017, 51, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Zysset, P.K.; Dall’Ára, E.; Varga, P. Finite element analysis for prediction of bone strength. Bonekey Rep. 2013, 2, 386. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.; Smith, P.A.; Eckstein, E.C.; Harris, G.F. Mechanical properties of OI type III bone tissue measured by nanoindentation. J. Biomed. Mater. Res. A 2006, 79, 71–77. [Google Scholar] [CrossRef]
- Weber, M.; Roschger, P.; Fratzl-Zelman, N.; Schöberl, T.; Rauch, F.; Glorieux, F.H.; Fratzl, P.; Klaushofer, K. Pamidronate does not adversely affect bone intrinsic material properties in children with osteogenesis imperfecta. Bone 2006, 39, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Smith, P.A.; Harris, G.F.; Rauch, F.; Bajorunaite, R. Comparison of nanoindentation measurements between osteogenesis imperfecta Type III and Type IV and between different anatomic locations (femur/tibia versus iliac crest). Connect Tissue Res. 2007, 48, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Smith, P.; Rauch, F.; Harris, G.F. Nanoindentation as a means for distinguishing clinical type of osteogenesis imperfect. Compos. Part B Eng. 2007, 38, 411–415. [Google Scholar] [CrossRef]
- Albert, C.; Jameson, J.; Toth, J. Bone Properties by Nanoindentation in Mild and Severe Osteogenesis Imperfecta. Clin. Biomech. 2013, 28, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.; Jameson, J.; Smith, P.; Harris, G. Reduced diaphyseal strength associated with high intracortical vascular porosity within long bones of children with osteogenesis imperfecta. Bone 2014, 66, 121–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imbert, L.; Aurégan, J.C.; Pernelle, K.; Hoc, T. Mechanical and mineral properties of osteogenesis imperfecta human bones at the tissue level. Bone 2014, 65, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Vardakastani, V.; Saletti, D.; Skalli, W.; Marry, P.; Allain, J.M.; Adam, C. Increased intra-cortical porosity reduces bone stiffness and strength in pediatric patients with osteogenesis imperfecta. Bone 2014, 69, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Imbert, L.; Aurégan, J.; Pernelle, K. Microstructure and compressive mechanical properties of cortical bone in children with osteogenesis imperfecta treated with bisphosphonates compared with healthy children. J. Mech. Behav. Biomed. Mater. 2015, 46, 261–270. [Google Scholar] [CrossRef] [PubMed]




















| Mechanical Property | Tissue | |
|---|---|---|
| Cortical | Cortical | |
| Modulus of elasticity transversal | 5764.54 MPa | 793.88 MPa |
| Modulus of elasticity longitudinal | 3627.78 MPa | 449.41 MPa |
| Shear modulus | 2097.44 MPa | 217.40 MPa |
| Shear modulus | 1450.43 MPa | 184.79 MPa |
| Poisson’s ratio | 0.27 | 0.21 |
| Author | Year | Type OI | Method of Obtaining | Cortical E (GPa) | Trabecular E (GPa) |
|---|---|---|---|---|---|
| Fan et al. [45] | 2006 | III | Nanoindentation | 15.22 (L) 13.92 (T) | 13.60 |
| Weber et al. [46] | 2006 | III–IV | Nanoindentation | 21.3 | |
| Fan et al. [47] | 2007 | III | Nanoindentation | 19.19 | 18.56 |
| Fan et al. [48] | 2007 | III | Nanoindentation | 19.67 | 19.23 |
| Albert et al. [49] | 2013 | III | Nanoindentation | 16.3 | |
| Albert et al. [50] | 2014 | IV | Mechanical tests | 4.4 (L) 1.6 (T) | |
| Imbert et al. [51] | 2014 | − | Nanoindentation | 17.6 | |
| Vardakastani et al. [52] | 2014 | − | Mechanical tests | 6.8 | |
| Imbert et al. [53] | 2015 | − | Mechanical tests | 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Vela, V.; Aguilar-Pérez, L.A.; Paredes-Rojas, J.C.; Flores-Campos, J.A.; Ortiz-Hernández, F.E.; Torres-SanMiguel, C.R. Bone Fractures Numerical Analysis in a Femur Affected by Osteogenesis Imperfecta. Children 2021, 8, 1177. https://doi.org/10.3390/children8121177
Ramírez-Vela V, Aguilar-Pérez LA, Paredes-Rojas JC, Flores-Campos JA, Ortiz-Hernández FE, Torres-SanMiguel CR. Bone Fractures Numerical Analysis in a Femur Affected by Osteogenesis Imperfecta. Children. 2021; 8(12):1177. https://doi.org/10.3390/children8121177
Chicago/Turabian StyleRamírez-Vela, Viridiana, Luis Antonio Aguilar-Pérez, Juan Carlos Paredes-Rojas, Juan Alejandro Flores-Campos, Fernando ELi Ortiz-Hernández, and Christopher René Torres-SanMiguel. 2021. "Bone Fractures Numerical Analysis in a Femur Affected by Osteogenesis Imperfecta" Children 8, no. 12: 1177. https://doi.org/10.3390/children8121177