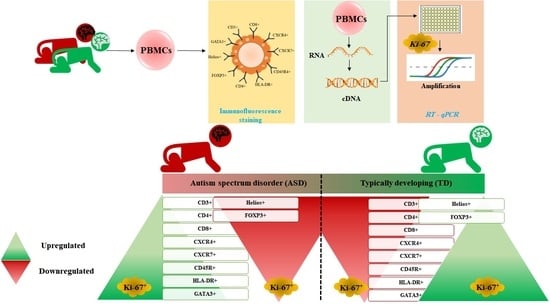
Dysregulation of Ki-67 Expression in T Cells of Children with Autism Spectrum Disorder
Abstract
:1. Introduction
2. Material Methods
2.1. Ethics Approval
2.2. Study Participants
2.3. Study Measurements
2.4. Chemicals and Antibodies
2.5. Flow Cytometric Analysis
2.6. Gene Expression
2.7. Statistics
3. Results
3.1. Increased Ki-67 Expression in T Cell Surface Receptor+ Cells in Children with ASD
3.2. Increased Ki-67 Expression in CXCR4 and CXCR7+ Cells in Children with ASD
3.3. Ki-67 Production Is Upregulated in CD45R+ and HLA-DR+ Cells in Children with ASD
3.4. GATA3, Helios, and FOXP3 Transcription Factor Expression in Ki-67 Producing Cells in Children with ASD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Betancur, C. Etiological heterogeneity in autism spectrum disorders: More than 100 genetic and genomic disorders and still counting. Brain Res. 2011, 1380, 42–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2015. [Google Scholar]
- Careaga, M.; Rogers, S.; Hansen, R.L.; Amaral, D.G.; Van de Water, J.; Ashwood, P. Immune Endophenotypes in Children With Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 434–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mead, J.; Ashwood, P. Evidence supporting an altered immune response in ASD. Immunol. Lett. 2015, 163, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Goines, P.; Van de Water, J. The immune system’s role in the biology of autism. Curr. Opin. Neuro.l 2010, 23, 111–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meltzer, A.; Van de Water, J. The role of the immune system in autism spectrum disorder. Neuropsychopharmacology 2017, 42, 284–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zerbo, O.; Leong, A.; Barcellos, L.; Bernal, P.; Fireman, B.; Croen, L.A. Immune mediated conditions in autism spectrum disorders. Brain Behav. Immun. 2015, 46, 232–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.; Van de Water, J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011, 25, 40–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mélik-Parsadaniantz, S.; Rostène, W. Chemokines and neuromodulation. J. Neuroimmunol. 2008, 198, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.F.; Nadeem, A.; Ansari, M.A.; Bakheet, S.A.; Attia, S.M.; Zoheir, K.M.; Al-Ayadhi, L.Y.; Alzahrani, M.Z.; Alsaad, A.M.; Alotaibi, M.R.; et al. Imbalance between the anti- and pro-inflammatory milieu in blood leukocytes of autistic children. Mol. Immunol. 2017, 82, 57–65. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Zoheir, K.M.A.; Ansari, M.A.; Nadeem, A.; Bakheet, S.A.; Al-Ayadhi, L.Y.; Alzahrani, M.Z.; Al-Shabanah, O.A.; Al-Harbi, M.M.; Attia, S.M. Dysregulation of Th1, Th2, Th17, and T regulatory cell-related transcription factor signaling in children with autism. Mol. Neurobiol. 2017, 54, 4390–4400. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.F.; Nadeem, A.; Ansari, M.A.; Bakheet, S.A.; Al-Ayadhi, L.Y.; Attia, S.M. Upregulation of IL-9 and JAK-STAT signaling pathway in children with autism. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Vargas, D.L.; Nascimbene, C.; Knshnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.W.; Larsen, N.; Grove, J.; Nørgaard-Pedersen, B.; Thorsen, P.; Mortensen, E.L.; Hougaard, D.M. Amniotic fluid chemokines and autism spectrum disorders: An exploratory study utilizing a Danish Historic Birth Cohort. Brain Behav. Immun. 2012, 26, 170–176. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Ansari, M.A.; Nadeem, A.; Bakheet, S.A.; Al-Ayadhi, L.Y.; Attia, S.M. Upregulation of peripheral CXC and CC chemokine receptor expression on CD4+ T cells is associated with immune dysregulation in children with autism. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 81, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Jyonouchi, H.; Sun, S.; Le, H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J. Neuroimmunol. 2001, 120, 170–179. [Google Scholar] [CrossRef]
- Enstrom, A.M.; Onore, C.E.; Van de Water, J.A.; Ashwood, P. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav. Immun. 2010, 24, 64–71. [Google Scholar] [CrossRef] [Green Version]
- López-Cacho, J.M.; Gallardo, S.; Posada, M.; Aguerri, M.; Calzada, D.; Mayayo, T.; Lahoz, C.; Cárdaba, B. Characterization of immune cell phenotypes in adults with autism spectrum disorders. J. Investig. Med. 2016, 64, 1179–1185. [Google Scholar] [CrossRef]
- Lee, L.C.; Zachary, A.A.; Leffell, M.S.; Newschaffer, C.J.; Matteson, K.J.; Tyler, J.D.; Zimmerman, A.W. HLA-DR4 in families with autism. Pediatr. Neurol. 2006, 35, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.R.; Maciulis, A.; Stubbs, E.G.; Cutler, A.; Odell, D. The transmission disequilibrium test suggests that HLA-DR4 and DR13 are linked to autism spectrum disorder. Hum. Immunol. 2002, 63, 311–316. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Chow, J.; Mazmanian, S.K.; Patterson, P.H. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc. Natl. Acad. Sci. USA 2012, 109, 12776–12781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.; Ding, M.; Xu, K.; Li, L.; Mao, L.; Zheng, J. Ki67 targeted strategies for cancer therapy. Clin. Transl. Oncol. 2018, 20, 570–575. [Google Scholar] [CrossRef]
- Ishihara, M.; Mukai, H.; Nagai, S.; Onozawa, M.; Nihei, K.; Shimada, T.; Wada, N. Retrospective analysis of risk factors for central nervous system metastases in operable breast cancer: Effects of biologic subtype and Ki67 overexpression on survival. Oncology 2013, 84, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Pearson, B.L.; Corley, M.J.; Vasconcellos, A.; Blanchard, D.C.; Blanchard, R.J. Heparan sulfate deficiency in autistic postmortem brain tissue from the subventricular zone of the lateral ventricles. Behav. Brain Res. 2013, 243, 138–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsarovina, K.; Pattyn, A.; Stubbusch, J.; Müller, F.; van der Wees, J.; Schneider, C.; Brunet, J.F.; Rohrer, H. Essential role of Gata transcription factors in sympathetic neuron development. Development 2004, 131, 4775–4786. [Google Scholar] [CrossRef] [Green Version]
- Rout, U.K.; Clausen, P. Common increase of GATA-3 level in PC-12 cells by three teratogens causing autism spectrum disorders. Neurosci. Res. 2009, 64, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Schopler, E.; Reichler, R.J.; Renner, B.R. The Childhood Autism Rating Scale (CARS) for Diagnostic Screening and Classification in Autism; Irvington: New York, NY, USA, 1986. [Google Scholar]
- Noster, R.; Riedel, R.; Mashreghi, M.F.; Radbruch, H.; Harms, L.; Haftmann, C.; Chang, H.-D.; Radbruch, A.; Zielinski, C.E. IL-17 and GM-CSF expression are antagonistically regulated by human T helper cells. Sci. Transl. Med. 2014, 6, 241ra80. [Google Scholar] [CrossRef]
- Ormstad, H.; Bryn, V.; Saugstad, O.D.; Skjeldal, O.; Maes, M. Role of the Immune System in Autism Spectrum Disorders (ASD) CNS & Neurological Disorders-Drug Targets. Former. Curr. Drug Targets CNS Neurol. Disord. 2018, 17, 489–495. [Google Scholar]
- Onore, C.; Careaga, M.; Ashwood, P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav. Immun. 2012, 26, 383–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masi, A.; Quintana, D.S.; Glozier, N.; Lloyd, A.R.; Hickie, I.B.; Guastella, A.J. Cytokine aberrations in autism spectrum disorder: A systematic review and meta-analysis. Mol. Psychiatry 2015, 20, 440–446. [Google Scholar] [CrossRef]
- Gładysz, D.; Krzywdzińska, A.; Hozyasz, K.K. Immune abnormalities in autism spectrum disorder-could they hold promise for causative treatment? Mol. Neurobiol. 2018, 55, 6387–6435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaman, S.; Yazdani, U.; Deng, Y. A search for blood biomarkers for autism: Peptoids. Sci. Rep. 2016, 6, 19164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depino, A.M. Peripheral and central inflammation in autism spectrum disorders. Mol. Cell Neurosci. 2013, 53, 69–76. [Google Scholar] [CrossRef]
- Matta, S.M.; Hill-Yardin, E.L.; Crack, P.J. The influence of neuroinflammation in Autism Spectrum Disorder. Brain Behav. Immun. 2019, 79, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Pei, D.S.; Qian, G.W.; Tian, H.; Mou, J.; Li, W.; Zheng, J.N. Analysis of human Ki-67 gene promoter and identification of the Sp1 binding sites for Ki-67 transcription. Tumour. Biol. 2012, 33, 257–266. [Google Scholar] [CrossRef]
- Pessler, F.; Ogdie, A.; Diaz-Torne, C.; Dai, L.; Yu, X.; Einhorn, E.; Gay, S.; Schumacher, H.R. Subintimal Ki-67 as a synovial tissue biomarker for inflammatory arthropathies. Ann. Rheum. Dis. 2008, 67, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Balashov, K.E.; Rottman, J.B.; Weiner, H.L.; Hancock, W.W. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc. Natl. Acad. Sci. USA 1999, 96, 6873–6878. [Google Scholar] [CrossRef] [Green Version]
- Murdoch, C.; Monk, P.N.; Finn, A. Functional expression of chemokine receptor CXCR4 on human epithelial cells. Immunology 1999, 98, 36–41. [Google Scholar] [CrossRef]
- Li, Y.; Tang, G.; Liu, Y.; He, X.; Huang, J.; Lin, X.; Zhang, Z.; Yang, G.-Y.; Wang, Y. CXCL12 Gene Therapy Ameliorates Ischemia-Induced White Matter Injury in Mouse Brain. Stem. Cells Transl. Med. 2015, 4, 1122–1130. [Google Scholar] [CrossRef]
- Schonemeier, B.; Kolodziej, A.; Schulz, S.; Jacobs, S.; Hoellt, V.; Stumm, R. Regional and cellular localization of the CXCl12/SDF-1 chemokine receptor CXCR7 in the developing and adult rat brain. J. Comp. Neurol. 2008, 510, 207–220. [Google Scholar] [CrossRef]
- Comi, A.M.; Zimmerman, A.W.; Frye, V.H.; Law, P.A.; Peeden, J.N. Familial clustering of autoimmune disorders and evaluation of medical risk factors in autism. J. Child Neurol. 1999, 14, 388–394. [Google Scholar] [CrossRef]
- Levin, L.; Ban, Y.; Concepcion, E.; Davies, T.F.; Greenberg, D.A.; Tomer, Y. Analysis of HLA genes in families with autoimmune diabetes and thyroiditis. Hum. Immunol. 2004, 65, 640–647. [Google Scholar] [CrossRef]
- Johnson, W.G.; Buyske, S.; Mars, A.E.; Sreenath, M.; Stenroos, E.S.; Williams, T.A.; Stein, R.; Lambert, G.H. HLA-DR4 as a risk allele for autism acting in mothers of probands possibly during pregnancy. Arch. Pediatr. Adolesc. Med. 2009, 163, 542–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Doorninck, J.H.; van Der Wees, J.; Karis, A.; Goedknegt, E.; Coesmans, M.; Rutteman, M.; Grosveld, F.; De Zeeuw, C.I. GATA-3 is involved in the development of serotonergic neurons in the caudal raphe nuclei. J. Neurosci. 1999, 19, RC12. [Google Scholar] [CrossRef]
- Hong, S.J.; Choi, H.J.; Hong, S.; Huh, Y.; Chae, H.; Kim, K.-S. Transcription factor GATA-3 regulates the transcriptional activity of dopamine β-hydroxylase by interacting with Sp1 and AP4. Neurochem. Res. 2008, 33, 1821–1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.Y.; Li, Z.Y.; Zou, H.L.; Hu, Z.L.; Song, N.N.; Zheng, M.H.; Su, C.J.; Ding, Y.Q. Expression of the transcription factor GATA3 in the postnatal mouse central nervous system. Neurosci. Res. 2008, 61, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Hjort, M.; Thorvaldson, L.; Sandler, S. Concomitant analysis of Helios and Neuropilin-1 as a marker to detect thymic derived regulatory T cells in naive mice. Sci. Rep. 2015, 5, 7767. [Google Scholar] [CrossRef] [Green Version]
- Ross, E.M.; Bourges, D.; Hogan, T.V.; Gleeson, P.A.; van Driel, I.R. Helios defines T cells being driven to tolerance in the periphery and thymus. Eur. J. Immunol. 2014, 44, 2048–2058. [Google Scholar] [CrossRef]
- Golding, A.; Hasni, S.; Illei, G.; Shevach, E.M. The percentage of FoxP3+Helios+ Treg cells correlates positively with disease activity in systemic lupus erythematosus. Arthritis Rheum. 2013, 65, 2898–2906. [Google Scholar] [CrossRef] [Green Version]
- Safari, M.R.; Ghafouri-Fard, S.; Noroozi, R.; Sayad, A.; Omrani, M.D.; Komaki, A.; Eftekharian, M.M.; Taheri, M. FOXP3 gene variations and susceptibility to autism: A case-control study. Gene 2017, 596, 119–122. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhosaini, K.; Ansari, M.A.; Nadeem, A.; Attia, S.M.; Bakheet, S.A.; Al-Ayadhi, L.Y.; Mahmood, H.M.; Al-Mazroua, H.A.; Ahmad, S.F. Dysregulation of Ki-67 Expression in T Cells of Children with Autism Spectrum Disorder. Children 2021, 8, 116. https://doi.org/10.3390/children8020116
Alhosaini K, Ansari MA, Nadeem A, Attia SM, Bakheet SA, Al-Ayadhi LY, Mahmood HM, Al-Mazroua HA, Ahmad SF. Dysregulation of Ki-67 Expression in T Cells of Children with Autism Spectrum Disorder. Children. 2021; 8(2):116. https://doi.org/10.3390/children8020116
Chicago/Turabian StyleAlhosaini, Khaled, Mushtaq A. Ansari, Ahmed Nadeem, Sabry M. Attia, Saleh A. Bakheet, Laila Y. Al-Ayadhi, Hafiz M. Mahmood, Haneen A. Al-Mazroua, and Sheikh F. Ahmad. 2021. "Dysregulation of Ki-67 Expression in T Cells of Children with Autism Spectrum Disorder" Children 8, no. 2: 116. https://doi.org/10.3390/children8020116