The First Metabolome Analysis in Children with Epilepsy and ALG13-CDG Resulting from c.320A>G Variant
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Patients
- Patient 1
- Patient 2
- Patient 3
2.2. Metabolomic Analyses
- Scores plot (PCA and OPLS-DA)—used to observe the patterns or class separation in the data.
- S-plot (OPLS-DA)—giving insight into the variables’ importance for the observed class separation. The variables shifted away from the plot origin along the y axis (p-corr) had high reliability, while the variables more shifted along the x axis showed a greater magnitude of the changes. The ideal candidate for a biomarker has both high reliability and magnitude.
3. Results
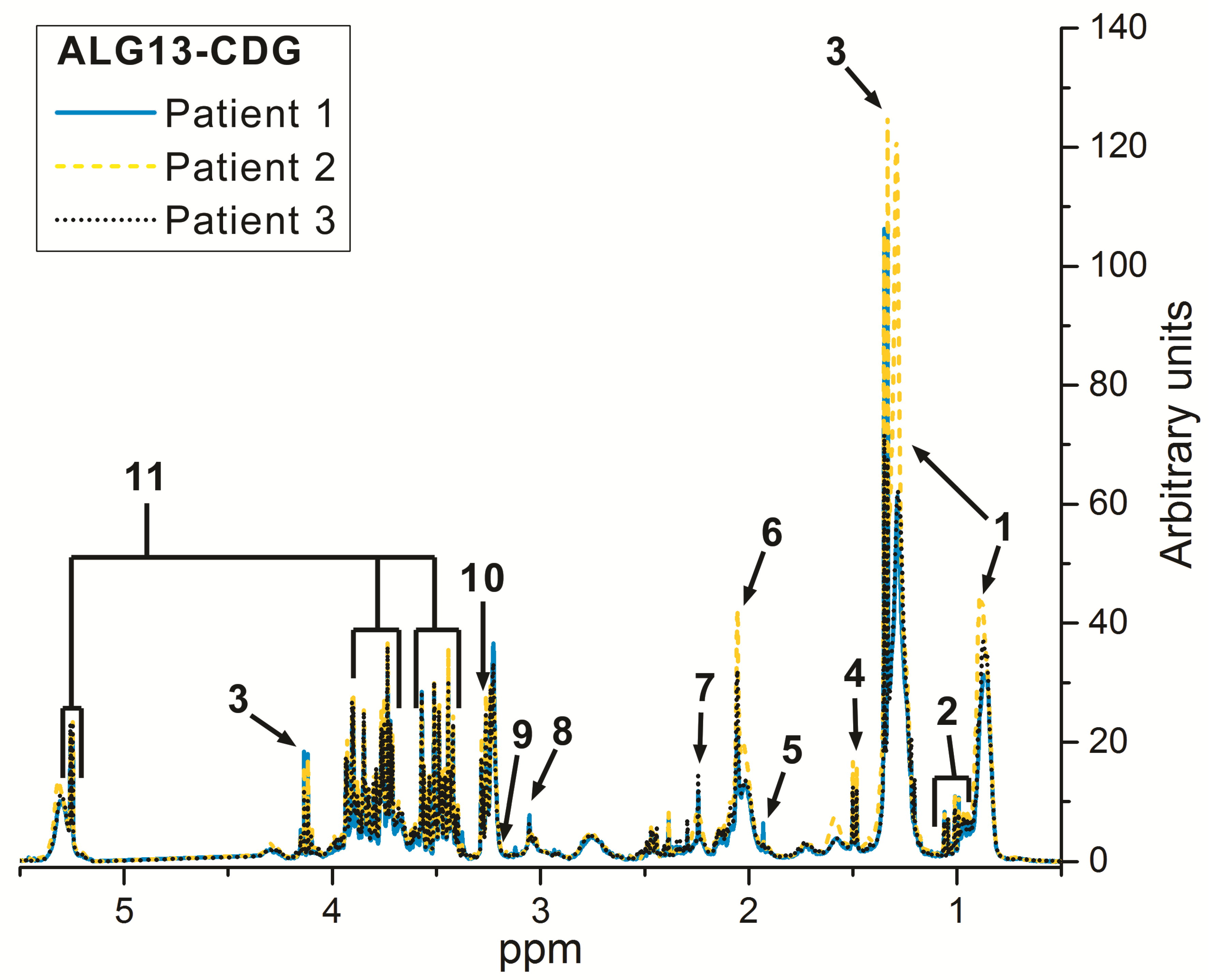
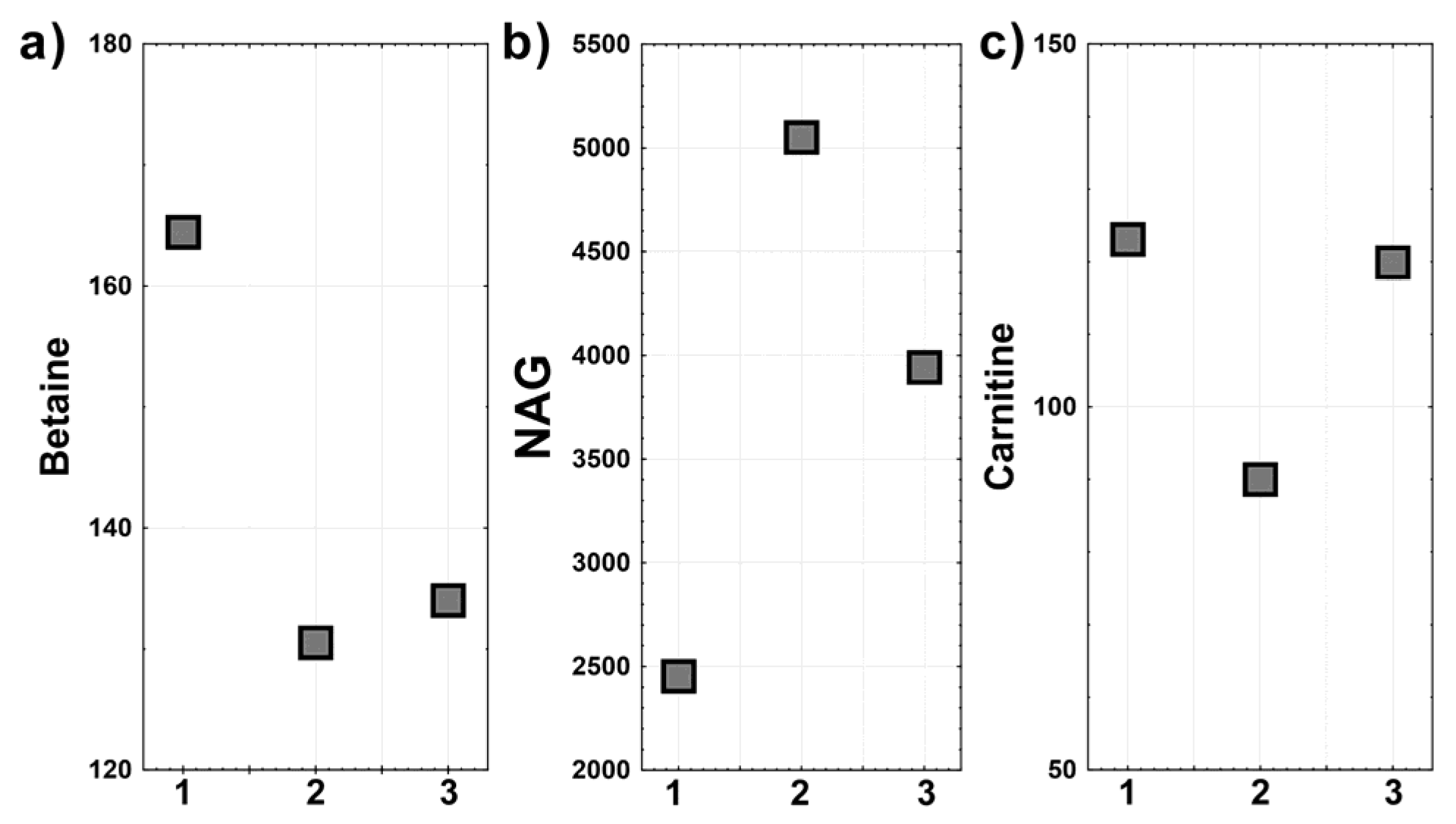
Metabolomic Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaeken, J.; Peanne, R. What is new in CDG? J. Inherit. Metab. Dis. 2017, 40, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Francisco, R.; Marques-da-Silva, D.; Brasil, S.; Pascoal, C.; Dos Reis Ferreira, V.; Morava, E.; Jaeken, J. The challenge of CDG diagnosis. Mol. Genet. Metab. 2019, 126, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.; Miao, H.; Lam, C. Congenital disorders of glycosylation. Ann. Transl. Med. 2018, 6, 477. [Google Scholar] [CrossRef] [PubMed]
- Péanne, R.; de Lonlay, P.; Foulquier, F.; Kornak, U.; Lefeber, D.J.; Morava, E.; Pérez, B.; Seta, N.; Thiel, C.; Van Schaftingen, E.; et al. Congenital disorders of glycosylation (CDG): Quo vadis? Eur. J. Med. Genet. 2018, 61, 643–663. [Google Scholar] [CrossRef]
- Pearl Phillip, L. Inherited Metabolic Epilepsies, 2nd ed.; Springer Publishing Company: New York, NY, USA, 2018; pp. 432–433. [Google Scholar]
- Gardeitchik, T.; Wyckmans, J.; Morava, E. Complex phenotypes in inborn errors of metabolism. Pediatr. Clin. N. Am. 2018, 65, 375–388. [Google Scholar] [CrossRef]
- Jezela-Stanek, A.; Bogdańska, A.; Pokora, P.; Paprocka, J.; Tylki-Szymańska, A. X-linked ALG13-CDG resulting from c.320A>G variant—can transferrin profile change and correlate with the clinical phenotype? Abstracts from the 53rd European Society of Human Genetics (ESHG) Conference: E-Posters. Eur. J. Hum. Genet. 2020, 28, 798–1016. [Google Scholar] [CrossRef]
- Hamici, S.; Bastaki, F.; Khalifa, M. Exome sequence identified a c.320A>G ALG13 variant in a female with infantile epileptic encephalopathy with normal glycosylation and random X inactivation: Review of the literature. Eur. J. Med. Genet. 2017, 60, 541–547. [Google Scholar] [CrossRef]
- Smith-Packard, B.; Myers, S.M.; Williams, M.S. Girls with Seizures Due to the c.320A>G Variant in ALG13 Do Not Show Abnormal Glycosylation Pattern on Standard Testing. JIMD Rep. 2015, 22, 95–98. [Google Scholar]
- Consortium, E.K. De Novo Mutations in SLC1A2 and CACNA1A Are Important Causes of Epileptic Encephalopathies. Am. J. Hum. Genet. 2016, 99, 287–298. [Google Scholar]
- Madaan, P.; Negi, S.; Sharma, R.; Kaur, A.; Sahu, J.K. X-Linked ALG13 Gene Variant as a Cause of Epileptic Encephalopathy in Girls. Indian J. Pediatr. 2019, 86, 1072–1073. [Google Scholar] [CrossRef]
- Galama, W.H.; Verhaagen-van den Akker, S.L.J.; Lefeber, D.J.; Feenstra, I.; Verrips, A. ALG13-CDG with Infantile Spasms in a Male Patient Due to a De Novo ALG13 Gene Mutation. JIMD Rep. 2018, 40, 11–16. [Google Scholar]
- Ng, B.G.; Eklund, E.A.; Shiryaev, S.A.; Dong, Y.Y.; Abbott, M.A.; Asteggiano, C.; Bamshad, M.J.; Barr, E.; Bernstein, J.A.; Chelakkadan, S.; et al. Predominant and novel de novo variants in 29 individuals with ALG13 deficiency: Clinical description, biomarker status, biochemical analysis, and treatment suggestions. J. Inherit. Metab. Dis. 2020, 18. [Google Scholar] [CrossRef]
- Datta, A.N.; Bahi-Buisson, N.; Bienvenu, T.; Buerki, S.E.; Gardiner, F.; Cross, J.H.; Heron, B.; Kaminska, A.; Korff, C.M.; Lepine, A.; et al. The phenotypic spectrum of X-linked, infantile onset ALG13-related developmental and epileptic encephalopathy. Epilepsia 2021, 2, 325–334. [Google Scholar] [CrossRef]
- Boguszewicz, Ł.; Jamroz, E.; Ciszek, M.; Emich-Widera, E.; Kijonka, M.; Banasik, T.; Skorupa, A.; Sokół, M. NMR-based metabolomics in pediatric drug resistant epilepsy—preliminary results. Sci. Rep. 2019, 9, 15035. [Google Scholar] [CrossRef] [Green Version]
- Boguszewicz, L.; Hajduk, A.; Mrochem-Kwarciak, J.; Skorupa, A.; Ciszek, M.; Heyda, A.; Składkowski, K.; Sokół, M. 1H NMR based metabolomic approach to monitoring of the head and neck cancer treatment toxicity. Metabolomics 2016, 12, 102. [Google Scholar] [CrossRef]
- Wiklund, S. Multivariate data analysis for Omics. Umetrics 2008. Umeå, Sweden. Available online: https://metabolomics.se/Courses/MVA/MVA%20in%20Omics_Handouts_Exercises_Solutions_Thu-Fri.pdf (accessed on 5 March 2021).
- Kobayashi, Y.; Tohyama, J.; Kato, M.; Akasaka, N.; Magara, S.; Kawashima, H.; Ohashi, T.; Shiraishi, H.; Nakashima, M.; Saitsu, H.; et al. High prevalence of genetic alterations in early-onset epileptic encephalopathies associated with infantile movement disorders. Brain Dev. 2016, 38, 285–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtsubo, K.; Marth, J.D. Glycosylation in cellular mechanisms of health and disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef] [Green Version]
- Kitteringham, N.R.; Jenkins, R.E.; Lane, C.S.; Elliott, V.L.; Park, B.K. Multiple reaction monitoring for quantitative biomarker analysis in proteomics and metabolomics. J. Chromatogr. B Analyt. Technol. BioMed Life Sci 2009, 877, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Lawler, P.R.; Akinkuolie, A.O.; Chandler, P.D.; Moorthy, M.V.; Vandenburgh, M.J.; Schaumberg, D.A.; Lee, I.M.; Glynn, R.J.; Ridker, P.M.; Buring, J.E.; et al. Circulating N-Linked Glycoprotein Acetyls and Longitudinal Mortality Risk. Circ. Res. 2016, 118, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in Inflammation: Mechanistic Aspects and Applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, S.A. Betaine in human nutrition. Am J. Clin. Nutr 2004, 80, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Ueland, P.M. Choline and betaine in health and disease. J. Inherit. Metab. Dis. 2011, 34, 3–15. [Google Scholar] [CrossRef]
- Lever, M.; Slow, S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin. Biochem. 2010, 43, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Schwahn, B.C.; Hafner, D.; Hohlfeld, T.; Balkenhol, N.; Laryea, M.D.; Wendel, U. Pharmacokinetics of oral betaine in healthy subjects and patients with homocystinuria. Br. J. Clin. Pharmacol. 2003, 55, 6–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, J.D.; Brown, J.C.; Nicholson, J.K.; Sadler, P.J. Assignment of resonances for ’acute-phase’ glycoproteins in high resolution proton NMR spectra of human blood plasma. FEBS Lett. 1987, 215, 311–315. [Google Scholar] [CrossRef] [Green Version]
- Bremer, J. Carnitine-metabolism and functions. Physiol. Rev. 1983, 63, 1420–1480. [Google Scholar] [CrossRef]
- Bene, J.; Hadzsiev, K.; Melegh, B. Role of carnitine and its derivatives in the development and management of type 2 diabetes. Nutr. Diabetes 2018, 8, 8. [Google Scholar] [CrossRef]
- Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.D.; Garrett, Q. Role of carnitine in disease. Nutr. Metab. (Lond.) 2010, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- de Koning, T.J. Amino acid synthesis deficiencies. J. Inherit. Metab. Dis. 2017, 40, 609–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamari, F.; Mochel, F.; Saudubray, J.M. An overview of inborn errors of complex lipid biosynthesis and remodelling. J. Inherit. Metab. Dis. 2015, 38, 3–18. [Google Scholar] [CrossRef] [PubMed]
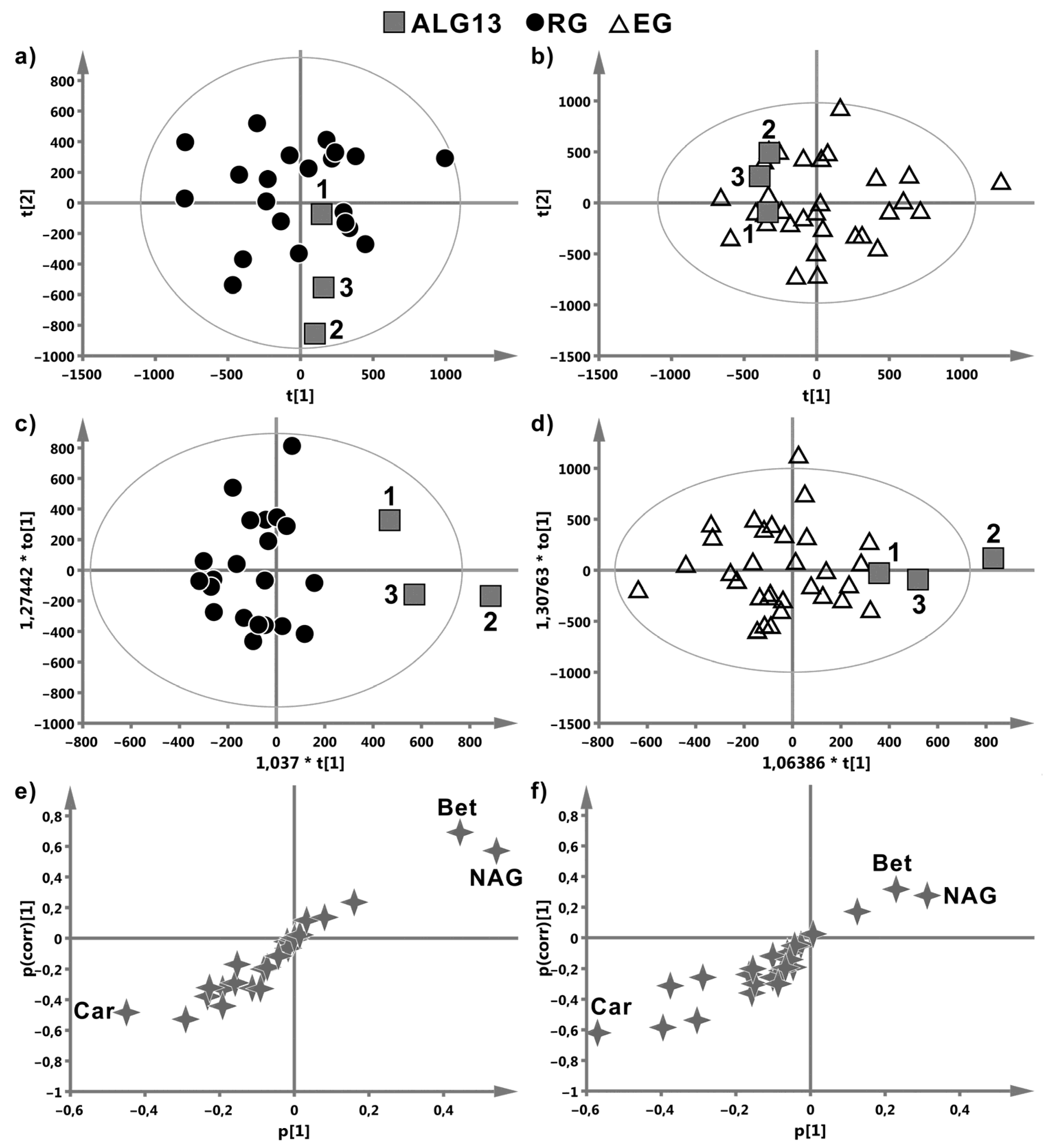

| ALG13 vs. RG | ALG13 vs. EG | ||||||
|---|---|---|---|---|---|---|---|
| Principal Component Analysis (PCA) | |||||||
| Principal Component | R2X (%) | Q2 | Principal Component | R2X (%) | Q2 | ||
| PC1 | 28 | 0.004 | PC1 | 22.6 | 0.044 | ||
| PC2 | 20.9 | 0.045 | PC2 | 18.2 | 0.071 | ||
| Orthogonal partial least squares—discriminant analysis (OPLS-DA) | |||||||
| Predictive component | R2X (%) | R2 | Q2 | Predictive component | R2X (%) | R2 | Q2 |
| P1 | 12.3 | 0.796 | 0.356 | P1 | 10.2 | 0.407 | −0.32 |
| Orthogonal component | R2X(o) (%) | Orthogonal component | R2X(o) (%) | ||||
| O1 | 18.5 | O1 | 18.8 | ||||
| O2 | 22.7 | ||||||
| cv-ANOVA for OPLS-DA model | p-value = 0.08 | cv-ANOVA for OPLS-DA model | p-value = >0.999 | ||||
| p-Values from Kruskal–Wallis ANOVA | |||
|---|---|---|---|
| Betaine | |||
| EG | RG | ALG13 | |
| EG | 0.186 | 0.132 | |
| RG | 0.014 | ||
| ALG13 | 0.186 | 0.014 | |
| NAG | |||
| EG | RG | ALG13 | |
| EG | 0.175 | >0.999 | |
| RG | 0.175 | 0.363 | |
| ALG13 | >0.999 | 0.363 | |
| Carnitine | |||
| EG | RG | ALG13 | |
| EG | 0.251 | 0.655 | |
| RG | 0.251 | 0.13 | |
| ALG13 | 0.655 | 0.13 | |
| Carnitine after removal of 1 RG case | |||
| EG | 0.103 | 0.62 | |
| RG | 0.103 | 0.074 | |
| ALG13 | 0.62 | 0.074 | |
| Kobayashi et al. 2016 [18] | Madaan et al. 2019 [11] | Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|---|---|
| Age/gender | Female | 30 mo | 2 yrs/female | 4 yrs/female | 5 yrs/female |
| Family history | not reported | not reported | unremarkable | unremarkable | recurrent ischemic strokes, also in the mother 2 times before pregnancy |
| Gestation (G) and delivery period (D) | not reported | not reported | GII, DII (Cesarean section, of maternal indication) birth weight—2430 g OFC—32 cm birth length—50 cm 10 points in Apgar scale | GII, DII, 42nd week of gestation (green amniotic fluid), 10 points in Apgar scale, normal birth parameters | GII (after months of efforts; DI—miscarriage), complicated by gestational diabetes mellitus, Cesarean section at term, normal birth parameters |
| Seizures’ morphology | 6 mo— epileptic spasms | 5 mo— epileptic spasms | 4 weeks—epileptic spasms | 4 mo—tonic seizures, epileptic spasms | 4 mo—epileptic spasms, myoclonic seizures, tonic seizures, partial seizures |
| EEG | 6 mo—hypsarrthythmia | 5 mo— hypsarrthythmia | 4 weeks- hypsarrthythmia 2 yrs—few generalized paroxysmal changes | 4mo— hypsarrthythmia | 4 mo—hypsarrthythmia, 5 yrs—partial (L>R) and generalized paroxysmal discharges |
| Drugs | ACTH | ACTH with epileptic spasms regression for 18mo | Vigabatrin, valproic acid, ACTH at present: valproic acid, reduction vigabatrin dosage | Vigabatrin, valproic acid, ACTH, topiramate, lamotrygine, at present: vigabatrin, lamotrygine | Valproic acid, levetiracetam, ACTH, topiramate, methylprednisolone at present: valproic acid, lamotrygine, topiramate, primidone |
| Psychomotor development | 3yrs—head control | generalized hypotonia, microcephaly, developmental delay | generalized hypotonia, microcephaly, developmental delay; can sit unsupported and crawl; good emotional contact | generalized hypotonia, microcephaly, developmental delay: can sit unsupported, try to walk by the hand; syllabize | generalized severe hypotonia (at 5 yrs is unable to control her head), OFC >97 c, multiple dystonic movements; from birth poor eye contact and lack of interest in the surrounding, 4 mo—noticeable decrease in spontaneous activity |
| Other complaints/diseases | chorea, dyskinesia | autistic features | astigmatism, strabismus and hyperopia; problems with chewing | tendency to autostimulation and autoaggression; synechia; astigmatism and hypermetropia | strabismus, facial dysmorphism—open-mouth appearance, high forehead, hypertelorism, short and up-turned nose, smooth philtrum and thin vermilion |
| Brain MRI | cerebral atrophy | normal | slightly delayed myelination of white matter | slight widening of the ventricular system (L>R) | normal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paprocka, J.; Jezela-Stanek, A.; Boguszewicz, Ł.; Sokół, M.; Lipiński, P.; Jamroz, E.; Emich-Widera, E.; Tylki-Szymańska, A. The First Metabolome Analysis in Children with Epilepsy and ALG13-CDG Resulting from c.320A>G Variant. Children 2021, 8, 251. https://doi.org/10.3390/children8030251
Paprocka J, Jezela-Stanek A, Boguszewicz Ł, Sokół M, Lipiński P, Jamroz E, Emich-Widera E, Tylki-Szymańska A. The First Metabolome Analysis in Children with Epilepsy and ALG13-CDG Resulting from c.320A>G Variant. Children. 2021; 8(3):251. https://doi.org/10.3390/children8030251
Chicago/Turabian StylePaprocka, Justyna, Aleksandra Jezela-Stanek, Łukasz Boguszewicz, Maria Sokół, Patryk Lipiński, Ewa Jamroz, Ewa Emich-Widera, and Anna Tylki-Szymańska. 2021. "The First Metabolome Analysis in Children with Epilepsy and ALG13-CDG Resulting from c.320A>G Variant" Children 8, no. 3: 251. https://doi.org/10.3390/children8030251
APA StylePaprocka, J., Jezela-Stanek, A., Boguszewicz, Ł., Sokół, M., Lipiński, P., Jamroz, E., Emich-Widera, E., & Tylki-Szymańska, A. (2021). The First Metabolome Analysis in Children with Epilepsy and ALG13-CDG Resulting from c.320A>G Variant. Children, 8(3), 251. https://doi.org/10.3390/children8030251








