Utility of Genetic Testing from the Perspective of Parents/Caregivers: A Scoping Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Synthesis
2.4. Quality Appraisal
3. Results
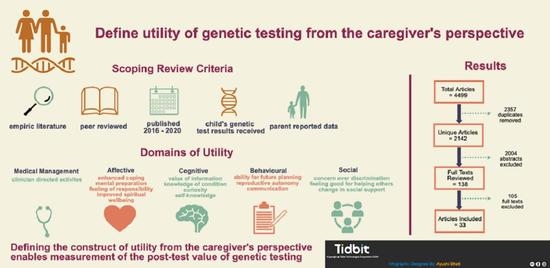
3.1. Study Selection, Characteristics and Quality
3.2. Utility from Parents’/Caregivers’ Perspectives
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johansen Taber, K.A.; Dickinson, B.D.; Wilson, M. The promise and challenges of next-generation genome sequencing for clinical care. JAMA Intern. Med. 2014, 174, 275–280. [Google Scholar] [CrossRef]
- Manolio, T.A.; Chisholm, R.L.; Ozenberger, B.; Roden, D.M.; Williams, M.S.; Wilson, R.; Bick, D.; Bottinger, E.P.; Brilliant, M.H.; Eng, C.; et al. Implementing genomic medicine in the clinic: The future is here. Genet. Med. 2013, 15, 258–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayeems, R.Z.; Boycott, K.M. Genome-wide sequencing technologies: A primer for paediatricians. Paediatr. Child Health 2017, 23, 191–197. [Google Scholar] [CrossRef]
- Boycott, K.M.; Hartley, T.; Adam, S.; Bernier, F.P.; Chong, K.; A Fernandez, B.; Friedman, J.M.; Geraghty, M.T.; Hume, S.; Knoppers, B.M.; et al. The clinical application of genome-wide sequencing for monogenic diseases in Canada: Position Statement of the Canadian College of Medical Geneticists. J. Med. Genet. 2015, 52, 431–437. [Google Scholar] [CrossRef]
- Cernat, A.; Hayeems, R.Z. Cascade health service use in family members following genetic testing in children: A scoping literature review. Eur. J. Hum. Genet. 2021. under review. [Google Scholar]
- Tsiplova, K.; Zur, R.M.; Marshall, C.R.; Stavropoulos, D.J.; Pereira, S.L.; Merico, D.; Young, E.J.; Sung, W.W.L.; Scherer, S.W.; Ungar, W.J. A microcosting and cost–consequence analysis of clinical genomic testing strategies in autism spectrum disorder. Genet. Med. 2017, 19, 1268–1275. [Google Scholar] [CrossRef] [Green Version]
- Caulfield, T.; Evans, J.P.; McGuire, A.L.; McCabe, C.; Bubela, T.; Cook-Deegan, R.; Fishman, J.R.; Hogarth, S.; Miller, F.A.; Ravitsky, V.; et al. Reflections on the Cost of “Low-Cost” Whole Genome Sequencing: Framing the Health Policy Debate. PLoS Biol. 2013, 11, e1001699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faulkner, E.; Holtorf, A.-P.; Walton, S.; Liu, C.Y.; Lin, H.; Biltaj, E.; Brixner, D.; Barr, C.; Oberg, J.; Shandhu, G.; et al. Being Precise About Precision Medicine: What Should Value Frameworks Incorporate to Address Precision Medicine? A Report of the Personalized Precision Medicine Special Interest Group. Value Health 2020, 23, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Hayeems, R.Z.; Dimmock, D.; Bick, D.; Belmont, J.W.; Green, R.C.; Lanpher, B.; Jobanputra, V.; Mendoza, R.; Kulkarni, S.; Grove, M.E.; et al. Clinical utility of genomic testing: A measurement toolkit. NPJ Genom. Med. 2020, 5, 56. [Google Scholar] [CrossRef]
- Strategy for Patient-Oriented Research (SPOR). Patient Engagement Framework. n.d. Available online: https://cihr-irsc.gc.ca/e/documents/spor_framework-en.pdf (accessed on 20 January 2021).
- Ma, J.O.R.; Wynn, J.; Biesecker, B.; Biesecker, L.G.; Ms, B.B.; Brothers, K.B.; Chung, W.K.; Christensen, K.D.; Green, R.C.; Jd, A.L.M.; et al. Psychological outcomes related to exome and genome sequencing result disclosure: A meta-analysis of seven Clinical Sequencing Exploratory Research (CSER) Consortium studies. Genet. Med. 2019, 21, 2781–2790. [Google Scholar] [CrossRef]
- Bunnik, E.M.; Janssens, A.C.J.W.; Schermer, M.H.N. Personal utility in genomic testing: Is there such a thing? J. Med. Ethics 2014, 41, 322–326. [Google Scholar] [CrossRef]
- Foster, M.W.; Mulvihill, J.J.; Sharp, R.R. Evaluating the utility of personal genomic information. Genet. Med. 2009, 11, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Lupo, P.J.; Robinson, J.O.; Diamond, P.M.; Jamal, L.; Danysh, H.E.; Blumenthal-Barby, J.; Lehmann, L.S.; Vassy, J.L.; Christensen, K.D.; Green, R.C.; et al. Patients’ perceived utility of whole-genome sequencing for their healthcare: Findings from the MedSeq project. Pers. Med. 2016, 13, 13–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayeems, R.Z.; Babul-Hirji, R.; Hoang, N.; Weksberg, R.; Shuman, C. Parents’ Experience with Pediatric Microarray: Transferrable Lessons in the Era of Genomic Counseling. J. Genet. Couns. 2015, 25, 298–304. [Google Scholar] [CrossRef]
- Kohler, J.N.; Turbitt, E.; Biesecker, B.B. Personal utility in genomic testing: A systematic literature review. Eur. J. Hum. Genet. 2017, 25, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Kohler, J.; Turbitt, E.; Lewis, K.; Wilfond, B.; Jamal, L.; Peay, H.; Biesecker, L.; Biesecker, B. Defining personal utility in genomics: A Delphi study. Clin. Genet. 2017, 92, 290–297. [Google Scholar] [CrossRef]
- Scheuner, M.T.; Russell, M.M.; Chanfreau-Coffinier, C.; Peredo, J.; Yano, E.M.; Hamilton, A.B.; Lerner, B.; Provenzale, D.; Knight, S.J.; Voils, C.I. Stakeholders’ views on the value of outcomes from clinical genetic and genomic interventions. Genet. Med. 2018, 21, 1371–1380. [Google Scholar] [CrossRef]
- Anderson, J.A.; Meyn, M.S.; Shuman, C.; Shaul, R.Z.; Mantella, L.E.; Szego, M.J.; Bowdin, S.; Monfared, N.; Hayeems, R.Z. Parents perspectives on whole genome sequencing for their children: Qualified enthusiasm? J. Med. Ethics 2016, 43, 535–539. [Google Scholar] [CrossRef]
- Dunsmore, K.; Gotlib Conn, L.; Zubairi, M.; Venkataramanan, V.; Liston, E.; Kim, R.; Hayeems, R.Z. Deciding to Pursue Pediatric Whole Genome Sequencing: Exploring the Values of Parents of Children with Cardiac Disease; American Society of Human Genetics (ASHG): Houston, TX, USA, 2019. [Google Scholar]
- Ungar, W.J. Challenges in health state valuation in paediatric economic evaluation: Are QALYs contraindicated? Pharmacoeconomics 2011, 29, 641–652. [Google Scholar] [CrossRef]
- Scapecchi, P. Valuation Differences between Adults and Children; Organisation for Economic Co-Operation and Development (OECD): Paris, France, 2006. [Google Scholar]
- Prosser, L.A. Current challenges and future research in measuring preferences for pediatric health outcomes. J. Pediatr. 2009, 155, 7–9. [Google Scholar] [CrossRef]
- Tricco, A.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Bramer, W.M.; Giustini, D.; de Jonge, G.B.; Holland, L.; Bekhuis, T. De-duplication of database search results for systematic reviews in EndNote. J. Med. Libr. Assoc. 2016, 104, 240–243. [Google Scholar] [CrossRef]
- World Health Organization. ICD-11—International Classification of Diseases, 11th Revision. Available online: https://icd.who.int/en (accessed on 20 July 2020).
- Kmet, L.; Lee, R.; Cook, L.S. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields; HTA Initiative #13. 2004; Alberta Heritage Foundation for Medical Research (AHFMR): Edmonton, AB, Canada, 2004. [Google Scholar]
- Hong, Q.; Pluye, P.; Fabregues, S.; Barltlett, G.; Boardman, F.K.; Cargo, M.; Dagenais, P.; Gagnon, M.P.; Griffiths, F.; Nicolau, B.; et al. Mixed Methods Appraisal Tool (MMAT) Version 2018: User Guide; McGill University: Montreal, QC, Canada, 2018. [Google Scholar]
- Gebhart, M.B.; Hines, R.S.; Penman, A.; Holland, A.C. How do patient perceived determinants influence the decision-making process to accept or decline preimplantation genetic screening? Fertil. Steril. 2016, 105, 188–193. [Google Scholar] [CrossRef] [Green Version]
- Chudleigh, J.; Buckingham, S.; Dignan, J.; O’Driscoll, S.; Johnson, K.; Rees, D.; Wyatt, H.; Metcalfe, A. Parents’ Experiences of Receiving the Initial Positive Newborn Screening (NBS) Result for Cystic Fibrosis and Sickle Cell Disease. J. Genet. Couns. 2016, 25, 1215–1226. [Google Scholar] [CrossRef] [Green Version]
- Szczepura, A.; Wynn, S.; Searle, B.; Khan, A.; Palmer, T.M.; Biggerstaff, D.; Elliott, J.; Hultén, M. UK families with children with rare chromosome disorders: Changing experiences of diagnosis and counselling (2003–2013). Clin. Genet. 2018, 93, 972–981. [Google Scholar] [CrossRef] [Green Version]
- Harrington, J.W.; Emuren, L.; Restaino, K.; Vergano, S.S. Parental Perception and Participation in Genetic Testing Among Children with Autism Spectrum Disorders. Clin. Pediatr. 2018, 57, 1642–1655. [Google Scholar] [CrossRef]
- Desai, P.; Haber, H.; Bulafka, J.; Russell, A.; Clifton, R.; Zachary, J.; Lee, S.; Feng, T.; Wapner, R.; Monk, C.; et al. Impacts of variants of uncertain significance on parental perceptions of children after prenatal chromosome microarray testing. Prenat. Diagn. 2018, 38, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.S.; Tabor, H.K.; Starks, H.; Garrison, N.A.; Laurino, M.; Burke, W. Pathways from autism spectrum disorder diagnosis to genetic testing. Genet. Med. 2018, 20, 737–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stivers, T.; Timmermans, S. The Actionability of Exome sequencing testing results. Sociol. Heal. Illn. 2017, 39, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Palomaki, G.E.; Kloza, E.M.; O’Brien, B.M.; Eklund, E.E.; Lambert-Messerlian, G.M. The clinical utility of DNA-based screening for fetal aneuploidy by primary obstetrical care providers in the general pregnancy population. Genet. Med. 2017, 19, 778–786. [Google Scholar] [CrossRef] [Green Version]
- Malek, J.; Slashinski, M.J.; Robinson, J.O.; Gutierrez, A.M.; Parsons, D.W.; Plon, S.E.; McCullough, L.B.; McGuire, A.L. Parental Perspectives on Whole-Exome Sequencing in Pediatric Cancer: A Typology of Perceived Utility. JCO Precis. Oncol. 2017, 1, 1–10. [Google Scholar] [CrossRef]
- Wilkins, E.J.; Archibald, A.D.; Sahhar, M.A.; White, S.M. “It wasn’t a disaster or anything”: Parents’ experiences of their child’s uncertain chromosomal microarray result. Am. J. Med. Genet. Part A 2016, 170, 2895–2904. [Google Scholar] [CrossRef]
- Vears, D.F.; Delany, C.; Massie, J.; Gillam, L. Parents’ experiences with requesting carrier testing for their unaffected children. Genet. Med. 2016, 18, 1199–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Steen, S.L.; Riedijk, S.R.; Verhagen-Visser, J.; Govaerts, L.C.; Srebniak, M.I.; Van Opstal, D.; Joosten, M.; Knapen, M.F.; Tibben, A.; Diderich, K.E.; et al. The Psychological Impact of Prenatal Diagnosis and Disclosure of Susceptibility Loci: First Impressions of Parents’ Experiences. J. Genet. Couns. 2016, 25, 1227–1234. [Google Scholar] [CrossRef] [Green Version]
- Rosell, A.M.; Pena, L.D.M.; Schoch, K.; Spillmann, R.; Sullivan, J.; Hooper, S.R.; Jiang, Y.-H.; Mathey-Andrews, N.; Goldstein, D.B.; Shashi, V. Not the End of the Odyssey: Parental Perceptions of Whole Exome Sequencing (WES) in Pediatric Undiagnosed Disorders. J. Genet. Couns. 2016, 25, 1019–1031. [Google Scholar] [CrossRef]
- Lingen, M.; Albers, L.; Borchers, M.; Haass, S.; Gärtner, J.; Schröder, S.; Goldbeck, L.; Von Kries, R.; Brockmann, K.; Zirn, B. Obtaining a genetic diagnosis in a child with disability: Impact on parental quality of life. Clin. Genet. 2015, 89, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Krabbenborg, L.; Vissers, L.E.L.M.; Schieving, J.; Kleefstra, T.; Kamsteeg, E.J.; Veltman, J.A.; Willemsen, M.A.; Van Der Burg, S. Understanding the Psychosocial Effects of WES Test Results on Parents of Children with Rare Diseases. J. Genet. Couns. 2016, 25, 1207–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krabbenborg, L.; Schieving, J.; Kleefstra, T.; Vissers, L.; Willemsen, M.; Veltman, J.; Van Der Burg, S. Evaluating a counselling strategy for diagnostic WES in paediatric neurology: An exploration of parents’ information and communication needs. Clin. Genet. 2015, 89, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Kerruish, N. Parents’ experiences 12 years after newborn screening for genetic susceptibility to type 1 diabetes and their attitudes to whole-genome sequencing in newborns. Genet. Med. 2015, 18, 249–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodgson, J.; Pitt, P.; Metcalfe, S.; Halliday, J.; Menezes, M.; Fisher, J.; Hickerton, C.; Petersen, K.; McClaren, B. Experiences of prenatal diagnosis and decision-making about termination of pregnancy: A qualitative study. Aust. N. Z. J. Obstet. Gynaecol. 2016, 56, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.L.; Rahm, A.K.; Zallen, D.T.; Stuckey, H.; Fultz, K.; Fan, A.L.; Bonhag, M.; Feldman, L.; Segal, M.M.; Williams, M.S. Impact of a Patient-Facing Enhanced Genomic Results Report to Improve Understanding, Engagement, and Communication. J. Genet. Couns. 2018, 27, 358–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansen Taber, K.A.; Beauchamp, K.A.; Lazarin, G.A.; Muzzey, D.; Arjunan, A.; Goldberg, J.D. Clinical utility of expanded carrier screening: Results-guided actionability and outcomes. Genet. Med. 2019, 21, 1041–1048. [Google Scholar] [CrossRef] [Green Version]
- Malek, J.; Pereira, S.; Robinson, J.O.; Gutierrez, A.M.; Slashinski, M.J.; Parsons, D.W.; Plon, S.E.; McGuire, A.L. Responsibility, culpability, and parental views on genomic testing for seriously ill children. Genet. Med. 2019, 21, 2791–2797. [Google Scholar] [CrossRef]
- Inglese, C.N.; Elliott, A.M.; Lehman, A. CAUSES Study New developmental syndromes: Understanding the family experience. J. Genet. Couns. 2019, 28, 202–212. [Google Scholar] [CrossRef]
- Wynn, J.; Ottman, R.; Duong, J.; Wilson, A.; Ahimaz, P.; Martinez, J.; Rabin, R.; Rosen, E.; Webster, R.; Au, C.; et al. Diagnostic exome sequencing in children: A survey of parental understanding, experience and psychological impact. Clin. Genet. 2018, 93, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Wou, K.; Weitz, T.; McCormack, C.; Wynn, J.; Spiegel, E.; Giordano, J.; Wapner, R.J.; Chung, W.K. Parental perceptions of prenatal whole exome sequencing (PPPWES) study. Prenat. Diagn. 2018, 38, 801–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldridge, C.E.; Osiovich, H.; Siden, H.; Elliott, A.M.; RAPIDOMICS Study; GenCOUNSEL Study. Rapid genome-wide sequencing in a neonatal intensive care unit: A retrospective qualitative exploration of parental experiences. J. Genet. Couns. 2020. [Google Scholar] [CrossRef]
- Berrios, C.; Koertje, C.; Noel-Macdonnell, J.; Soden, S.; Lantos, J. Parents of newborns in the NICU enrolled in genome sequencing research: Hopeful, but not naïve. Genet. Med. 2020, 22, 416–422. [Google Scholar] [CrossRef]
- Brett, G.R.; Martyn, M.; Lynch, F.; De Silva, M.G.; Ayres, S.; Gallacher, L.; Boggs, K.; Baxendale, A.; Schenscher, S.; King-Smith, S.; et al. Parental experiences of ultrarapid genomic testing for their critically unwell infants and children. Genet. Med. 2020, 22, 1976–1985. [Google Scholar] [CrossRef] [PubMed]
- Cakici, J.A.; Dimmock, D.P.; Caylor, S.A.; Gaughran, M.; Clarke, C.; Triplett, C.; Clark, M.M.; Kingsmore, S.F.; Bloss, C.S. A Prospective Study of Parental Perceptions of Rapid Whole-Genome and -Exome Sequencing among Seriously Ill Infants. Am. J. Hum. Genet. 2020, 107, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Luksic, D.; Sukhu, R.; Koval, C.; Cho, M.T.; Espinal, A.; Rufino, K.; Loarte, T.V.; Chung, W.K.; Wynn, J. A qualitative study of Latinx parents’ experiences of clinical exome sequencing. J. Genet. Couns. 2020, 29, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Mollison, L.; O’Daniel, J.M.; Henderson, G.E.; Berg, J.S.; Skinner, D. Parents’ perceptions of personal utility of exome sequencing results. Genet. Med. 2019, 22, 752–757. [Google Scholar] [CrossRef]
- Riggan, K.A.; Close, S.; Allyse, M.A. Family experiences and attitudes about receiving the diagnosis of sex chromosome aneuploidy in a child. Am. J. Med. Genet. Part C Semin. Med. Genet. 2020, 184, 404–413. [Google Scholar] [CrossRef]
- Sandow, R.; Kilpatrick, N.M.; Tan, T.Y.; Raj, S.; Forrest, L.E. Parental experiences and genetic counsellor roles in Pierre Robin sequence. J. Community Genet. 2020, 11, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.D.; Khoury, M.J. What is the clinical utility of genetic testing? Genet. Med. 2006, 8, 448–450. [Google Scholar] [CrossRef] [Green Version]
- Joseph, L.; Cankovic, M.; Caughron, S.; Chandra, P.; Emmadi, R.; Hagenkord, J.; Hallam, S.; Jewell, K.E.; Klein, R.D.; Pratt, V.M.; et al. The Spectrum of Clinical Utilities in Molecular Pathology Testing Procedures for Inherited Conditions and Cancer: A Report of the Association for Molecular Pathology. J. Mol. Diagn. 2016, 18, 605–619. [Google Scholar] [CrossRef]
- Centers for Disease Control. ACCE Model Process for Evaluating Genetic Tests 2010. Available online: https://www.cdc.gov/genomics/gtesting/acce/index.htm (accessed on 29 January 2021).
- Bossuyt, P.M.; Reitsma, J.B.; Linnet, K.; Moons, K.G. Beyond Diagnostic Accuracy: The Clinical Utility of Diagnostic Tests. Clin. Chem. 2012, 58, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Bruening, W.; Erinoff, E.; Schoelles, K.M. AHRQ Methods for Effective Health Care. Addressing Challenges in Genetic Test Evaluation: Evaluation Frameworks and Assessment of Analytic Validity; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2011.
- Hayeems, R.Z.; Luca, S.; Ungar, W.J.; Bhatt, A.; Chad, L.; Pullenayegum, E.; Meyn, M.S. The development of the Clinician-reported Genetic testing Utility InDEx (C-GUIDE): A novel strategy for measuring the clinical utility of genetic testing. Genet. Med. 2020, 22, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Buchanan, J.; Wordsworth, S. Evaluating the Outcomes Associated with Genomic Sequencing: A Roadmap for Future Research. PharmacoEconomics Open 2019, 3, 129–132. [Google Scholar] [CrossRef] [Green Version]
- Phillips, K.A.; Deverka, P.A.; Marshall, D.A.; Wordsworth, S.; Regier, D.A.; Christensen, K.D.; Buchanan, J. Methodological Issues in Assessing the Economic Value of Next-Generation Sequencing Tests: Many Challenges and Not Enough Solutions. Value Health 2018, 21, 1033–1042. [Google Scholar] [CrossRef] [Green Version]
- Payne, K.; Eden, M.; Davison, N.; Bakker, E. Toward health technology assessment of whole-genome sequencing diagnostic tests: Challenges and solutions. Pers. Med. 2017, 14, 235–247. [Google Scholar] [CrossRef]
- McAllister, M.; Wood, A.M.; Dunn, G.; Shiloh, S.; Todd, C. The Genetic Counseling Outcome Scale: A new patient-reported outcome measure for clinical genetics services. Clin. Genet. 2011, 79, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Grant, P.E.; Pampaka, M.; Payne, K.; Clarke, A.; McAllister, M. Developing a short-form of the Genetic Counselling Outcome Scale: The Genomics Outcome Scale. Eur. J. Med. Genet. 2019, 62, 324–334. [Google Scholar] [CrossRef] [PubMed]
- McConkie-Rosell, A.; Schoch, K.; Sullivan, J.; Cope, H.; Spillmann, R.; Palmer, C.G.; Pena, L.; Jiang, Y.; Daniels, N.; Walley, N.; et al. The genome empowerment scale: An assessment of parental empowerment in families with undiagnosed disease. Clin. Genet. 2019, 96, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.; Peltekova, I.; Savion-Lemieux, T.; Frei, J.; Bruno, R.; Joober, R.; Howe, J.; Scherer, S.W.; Elsabbagh, M. Perceived utility of biological testing for autism spectrum disorder is associated with child and family functioning. Res. Dev. Disabil. 2020, 100, 103605. [Google Scholar] [CrossRef]
- Li, M.; Bennette, C.S.; Amendola, L.M.; Hart, M.R.; Heagerty, P.; Comstock, B.; Tarczy-Hornoch, P.; Fullerton, S.M.; Regier, D.A.; Burke, W.; et al. The Feelings About genomiC Testing Results (FACToR) Questionnaire: Development and Preliminary Validation. J. Genet. Couns. 2019, 28, 477–490. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Luca, S.; Costain, G.; Snell, M.; Marano, M.; Curtis, M.; Dunsmore, K.; Veenma, D.; Walker, S.; Cohn, R.D.; et al. Genome sequencing among patients with medical complexity: What constitutes value? J. Genet. Couns. 2021. in preparation. [Google Scholar]
- Ungar, W.J. Economic Evaluation in Child Health; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- European Network for Health Technology Assessment (EUnetHTA). HTA Core Model Version 3.0 for the Full Assessment of Diagnostic Technologies, Medical and Surgical Interventions, Pharmaceuticals and Screening Technologies. EUnetHTA Joint Action 2, Work Package 8 2016. Available online: http://www.htacoremodel.info/Default.aspx (accessed on 4 February 2021).

| Studies Included | Count | % |
|---|---|---|
| 2020 | 8 | 24.2 |
| 2019 | 3 | 9.0 |
| 2018 | 7 | 21.2 |
| 2017 | 3 | 9.0 |
| 2016 | 12 | 36.4 |
| Characteristic | Count | |
| Country of Data Collection | ||
| USA | 18 | 54.5 |
| Europe | 6 | 18.2 |
| Australia | 5 | 15.2 |
| Canada | 3 | 9.1 |
| New Zealand | 1 | 3.0 |
| Population (Respondent Type) a | ||
| Parent/Caregiver of minor patient | 27 | 73.0 |
| Prospective Parent | 6 | 16.2 |
| Caregiver of adult patient | 3 | 8.1 |
| Adult sibling caregiver of minor patient | 1 | 2.7 |
| ICD-11 Disease Category a | ||
| Developmental anomalies | 20 | 60.6 |
| Mental, behavioural or neurodevelopmental disorders | 13 | 39.4 |
| Diseases of the nervous system | 7 | 21.2 |
| Prenatal or pre-implementation genetic testing b | 9 | 27.3 |
| Neoplasms | 2 | 6.1 |
| Diseases of the blood or blood-forming organs | 2 | 6.1 |
| Diseases of the respiratory system | 2 | 6.1 |
| Other c | 5 | 15.2 |
| Unreported | 9 | 27.3 |
| Genetic Test Type a | ||
| Karyotype, FISH, Fragile X, Single gene test | 12 | 36.4 |
| Chromosomal microarray | 16 | 48.5 |
| Multi-gene panel | 6 | 18.2 |
| Whole exome sequencing | 14 | 42.4 |
| Whole genome sequencing | 4 | 12.1 |
| Prenatal d | 4 | 12.1 |
| Newborn screening | 2 | 6.1 |
| Other e | 2 | 6.1 |
| Unreported | 1 | 3.0 |
| Study Type and Quality Scores | ||
| Qualitative | 20 | 60.6 |
| Average quality score (Range) | - | 81.0 (65.0–95.0) |
| Quantitative | 9 | 27.3 |
| Average quality score (Range) | - | 82.9 (64.0–95.5) |
| Mixed methods | 4 | 12.1 |
| Average quality score (Range) | - | 80.8 (75.0–92.3) |
| Total Primary Sample Size | 3606 | |
| Total Secondary Sample Size | 93 |
| Domain | Element | Element Concepts Genetic Test Results… | Number of Studies per Element | Percentage of Studies (N = 33) | Studies |
|---|---|---|---|---|---|
| Medical Management | Clinician-directed activities |
| 16 | 48.5% | 3–7, 9, 12, 13, 15, 16, 18, 19, 22, 26, 27, 28 |
| Affective | Enhanced coping |
| 14 | 42.4% | 2, 7, 11, 13, 17, 20–23, 26, 28, 30–32 |
| Mental Preparation |
| 19 | 57.6% | 4, 5, 7–10, 12, 17, 20–22, 24, 26–32 | |
| Feeling of responsibility |
| 24 | 72.7% | 3–5, 7–10, 12, 13, 15, 17, 20–24, 26–33 | |
| Improved Spiritual Wellbeing |
| 1 | 3.0% | 30 | |
| Cognitive | Value of Information |
| 12 | 36.4% | 9, 11, 14, 16–18, 21, 22, 26, 28, 29, 31 |
| Knowledge of Condition |
| 23 | 69.7% | 2–5, 7–9, 11–15, 17–20, 22, 23, 26, 27, 29–31 | |
| Curiosity |
| 8 | 24.2% | 1, 4, 7, 10, 21, 22, 30, 33 | |
| Self-Knowledge |
| 3 | 9.1% | 12, 17, 26 | |
| Behavioural | Ability for future planning |
| 9 | 27.3% | 4, 5, 8, 9, 12, 18, 26, 27, 31 |
| Reproductive Autonomy |
| 20 | 60.6% | 2–4, 7–9, 11, 13, 15, 18, 21, 22, 25–30, 32, 33 | |
| Communication |
| 8 | 24.2% | 1, 4, 5, 9, 17, 22, 26, 30 | |
| Social | Concern over discrimination |
| 7 | 21.2% | 1, 4, 10, 15, 22, 26, 30 |
| Feeling good for helping others |
| 3 | 9.1% | 15, 22, 31 | |
| Change in Social Support |
| 20 | 60.6% | 1, 3, 4, 7–9, 12, 15, 16, 18, 19, 21–23, 26, 30, 31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayeems, R.Z.; Luca, S.; Assamad, D.; Bhatt, A.; Ungar, W.J. Utility of Genetic Testing from the Perspective of Parents/Caregivers: A Scoping Review. Children 2021, 8, 259. https://doi.org/10.3390/children8040259
Hayeems RZ, Luca S, Assamad D, Bhatt A, Ungar WJ. Utility of Genetic Testing from the Perspective of Parents/Caregivers: A Scoping Review. Children. 2021; 8(4):259. https://doi.org/10.3390/children8040259
Chicago/Turabian StyleHayeems, Robin Z., Stephanie Luca, Daniel Assamad, Ayushi Bhatt, and Wendy J. Ungar. 2021. "Utility of Genetic Testing from the Perspective of Parents/Caregivers: A Scoping Review" Children 8, no. 4: 259. https://doi.org/10.3390/children8040259
APA StyleHayeems, R. Z., Luca, S., Assamad, D., Bhatt, A., & Ungar, W. J. (2021). Utility of Genetic Testing from the Perspective of Parents/Caregivers: A Scoping Review. Children, 8(4), 259. https://doi.org/10.3390/children8040259