Risk Factors of Growth Retardation and Developmental Deficits in Very Preterm Infants in a German Tertiary Neonatal Unit
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Developmental Care
2.3. Follow-Up Examination
2.4. Statistics
2.5. Ethics
3. Results
3.1. Patients

3.2. Risk-Factor-Related Outcomes
3.3. Developmental-Care-Related Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Shankaran, S.; Laptook, A.R.; Walsh, M.C.; Hale, E.C.; Newman, N.S.; Schibler, K.; Carlo, W.A.; et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010, 126, 443–456. [Google Scholar] [CrossRef]
- Serenius, F.; Källén, K.; Blennow, M.; Ewald, U.; Fellman, V.; Holmström, G.; Lindberg, E.; Lundqvist, P.; Maršál, K.; Norman, M.; et al. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. JAMA 2013, 309, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Horbar, J.D.; Carpenter, J.H.; Badger, G.J.; Kenny, M.J.; Soll, R.F.; Morrow, K.A.; Buzas, J.S. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics 2012, 129, 1019–1026. [Google Scholar] [CrossRef]
- Luttikhuizen dos Santos, E.S.; de Kieviet, J.F.; Königs, M.; van Elburg, R.M.; Oosterlaan, J. Predictive value of the Bayley scales of infant development on development of very preterm/very low birth weight children: A meta-analysis. Early Hum. Dev. 2013, 89, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Twilhaar, E.S.; Wade, R.M.; de Kieviet, J.F.; van Goudoever, J.B.; van Elburg, R.M.; Oosterlaan, J. Cognitive Outcomes of Children Born Extremely or Very Preterm Since the 1990s and Associated Risk Factors: A Meta-analysis and Meta-regression. JAMA Pediatr. 2018, 172, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Villamor-Martínez, E.; Pierro, M.; Cavallaro, G.; Mosca, F.; Villamor, E. Mother’s Own Milk and Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Front. Pediatr. 2019, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Spiegler, J.; Preuss, M.; Gebauer, C.; Bendiks, M.; Herting, E.; Gopel, W. Does Breastmilk Influence the Development of Bronchopulmonary Dysplasia? J. Pediatr. 2016, 169, 76–80.e4. [Google Scholar] [CrossRef]
- Young, L.; Embleton, N.D.; McGuire, W. Nutrient-enriched formula versus standard formula for preterm infants following hospital discharge. Cochrane Database Syst. Rev. 2016, 12. [Google Scholar] [CrossRef]
- Ferreira, R.C.; Mello, R.R.; Silva, K.S. Neonatal sepsis as a risk factor for neurodevelopmental changes in preterm infants with very low birth weight. J. Pediatr. 2014, 90, 293–299. [Google Scholar] [CrossRef]
- Beaino, G.; Khoshnood, B.; Kaminski, M.; Marret, S.; Pierrat, V.; Vieux, R.; Thiriez, G.; Matis, J.; Picaud, J.-C.; Rozé, J.-C.; et al. Predictors of the risk of cognitive deficiency in very preterm infants: The EPIPAGE prospective cohort. Acta Paediatr. 2011, 100, 370–378. [Google Scholar] [CrossRef]
- Ehrenkranz, R.A.; Younes, N.; Lemons, J.A.; Fanaroff, A.A.; Donovan, E.F.; Wright, L.L.; Katsikiotis, V.; Tyson, J.E.; Oh, W.; Shankaran, S.; et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics 1999, 104, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Klevebro, S.; Lundgren, P.; Hammar, U.; Smith, L.E.; Bottai, M.; Domellöf, M.; Löfqvist, C.; Hallberg, B.; Hellström, A. Cohort study of growth patterns by gestational age in preterm infants developing morbidity. BMJ Open 2016, 6, e012872. [Google Scholar] [CrossRef]
- de Kieviet, J.F.; Piek, J.P.; Aarnoudse-Moens, C.S.; Oosterlaan, J. Motor development in very preterm and very low-birth-weight children from birth to adolescence: A meta-analysis. JAMA 2009, 302, 2235–2242. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, A.; Yorifuji, T.; Hattori, M.; Tamai, K.; Nakamura, K.; Nakamura, M.; Kageyama, M.; Kubo, T.; Ogino, T.; Kobayashi, K.; et al. Catch-up growth and behavioral development among preterm, small-for-gestational-age children: A nationwide Japanese population-based study. Brain Dev. 2019, 41, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Altimier, L.B. Neuroprotective Core Measure 1: The Healing NICU Environment. Newborn Infant Nurs. Rev. 2015, 15, 91–96. [Google Scholar] [CrossRef]
- Vandormael, C.; Schoenhals, L.; Hüppi, P.S.; Filippa, M.; Borradori Tolsa, C. Language in Preterm Born Children: Atypical Development and Effects of Early Interventions on Neuroplasticity. Neural Plast. 2019, 2019. [Google Scholar] [CrossRef]
- Soleimani, F.; Azari, N.; Ghiasvand, H.; Shahrokhi, A.; Rahmani, N.; Fatollahierad, S. Do NICU developmental care improve cognitive and motor outcomes for preterm infants? A systematic review and meta-analysis. BMC Pediatr. 2020, 20, 67. [Google Scholar] [CrossRef] [PubMed]
- Taine, M.; Charles, M.-A.; Beltrand, J.; Roze, J.C.; Leger, J.; Botton, J.; Heude, B. Early postnatal growth and neurodevelopment in children born moderately preterm or small for gestational age at term: A systematic review. Paediatr. Perinat. Epidemiol. 2018, 32, 268–280. [Google Scholar] [CrossRef]
- Spittle, A.; Orton, J.; Anderson, P.J.; Boyd, R.; Doyle, L.W. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Developmental Follow-Up of Children and Young People Born Preterm; National Institute for Health and Care Excellence: London, UK, 2017. [Google Scholar]
- Ohlsson, A.; Jacobs, S.E. NIDCAP: A systematic review and meta-analyses of randomized controlled trials. Pediatrics 2013, 131, e881–e893. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Wurst, C.; Abele, H.; Hertzberg, C.; Peters, M.; Reuner, G.; Roll, C.; Rüdiger, M.; Trollmann, R.; Weißbrodt, A.; Wilken, B. S2k-Leitlinie 071-013: Sozialpädiatrische Nachsorge extrem unreifer Frühgeborener mit einem Geburtsgewicht unter 1000 Gramm. Available online: https://www.awmf.org/uploads/tx_szleitlinien/071-013l_S2k_Sozialpaed-Nachsorge-Fruehgeborener-unter-1000-g_2019-01.pdf (accessed on 13 December 2018).
- Bayley, N. Bayley Scales of Infant Development—Second Edition Manual, 2nd ed.; Deutsche Fassung; Harcourt Test Services: Frankfurt, Germany, 1993. [Google Scholar]
- Bayley, N. Bayley Scales of Infant Development, 2nd ed.; Harcourt Test Services GmbH: Heidelberg und Frankfurt, Germany, 2007. [Google Scholar]
- Reis, A.B.R.; de Mello, R.R.; Morsch, D.S.; Meio, M.D.B.B.; da Silva, K.S. Mental performance of very low birth weight preterm infants: Assessment of stability in the first two years of life and factors associated with mental performance. Rev. Bras. Epidemiol. 2012, 15, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Neuhauser, H.; Schienkiewitz, A.; Schaffrath Rosaria, A.; Dortschy, R.; Kurt, B.M. Referenzperzentile für anthropometrische Maßzahlen und Blutdruck aus der Studie zur Gesundheit von Kindern und Jugendlichen in Deutschland (KiGGS): 2. erweiterte Auflage. Dtsch. Natl. 2013. [Google Scholar]
- Villamor-Martínez, E.; Pierro, M.; Cavallaro, G.; Mosca, F.; Kramer, B.W.; Villamor, E. Donor Human Milk Protects against Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 238. [Google Scholar] [CrossRef]
- Pascal, A.; Govaert, P.; Oostra, A.; Naulaers, G.; Ortibus, E.; van den Broeck, C. Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: A meta-analytic review. Dev. Med. Child. Neurol. 2018, 60, 342–355. [Google Scholar] [CrossRef]
- Seppänen, A.-V.; Draper, E.S.; Petrou, S.; Barros, H.; Andronis, L.; Kim, S.W.; Maier, R.F.; Pedersen, P.; Gadzinowski, J.; Lebeer, J.; et al. Follow-up after very preterm birth in Europe. Arch. Dis. Child. Fetal Neonatal Ed. 2021. [Google Scholar] [CrossRef]
- Evensen, K.A.I.; Ustad, T.; Tikanmäki, M.; Haaramo, P.; Kajantie, E. Long-term motor outcomes of very preterm and/or very low birth weight individuals without cerebral palsy: A review of the current evidence. Semin. Fetal Neonatal Med. 2020, 25, 101116. [Google Scholar] [CrossRef]
- Torchin, H.; Morgan, A.S.; Ancel, P.-Y. International comparisons of neurodevelopmental outcomes in infants born very preterm. Semin. Fetal Neonatal Med. 2020, 25, 101109. [Google Scholar] [CrossRef]
- Lapillonne, A.; Bronsky, J.; Campoy, C.; Embleton, N.; Fewtrell, M.; Fidler Mis, N.; Gerasimidis, K.; Hojsak, I.; Hulst, J.; Indrio, F.; et al. Feeding the Late and Moderately Preterm Infant: A Position Paper of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 259–270. [Google Scholar] [CrossRef]
- Brown, J.V.E.; Walsh, V.; McGuire, W. Formula versus maternal breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2019, 8. [Google Scholar] [CrossRef]
- Henderson, G.; Fahey, T.; McGuire, W. Nutrient-enriched formula milk versus human breast milk for preterm infants following hospital discharge. Cochrane Database Syst. Rev. 2007. [Google Scholar] [CrossRef] [PubMed]
- Quigley, M.; Embleton, N.D.; McGuire, W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.E.; Sokol, J.; Ohlsson, A. The Newborn Individualized Developmental Care and Assessment Program is not supported by meta-analyses of the data. J. Pediatr. 2002, 140, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Redsell, S.A.; Glazebrook, C. Motor Development Interventions for Preterm Infants: A Systematic Review and Meta-analysis. Pediatrics 2016, 138. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Kane, A.E.; Brown, S.E.; Tarver, T.; Dusing, S.C. Effect of neonatal therapy on the motor, cognitive, and behavioral development of infants born preterm: A systematic review. Dev. Med. Child. Neurol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Little, A.A.; Kamholz, K.; Corwin, B.K.; Barrero-Castillero, A.; Wang, C.J. Understanding Barriers to Early Intervention Services for Preterm Infants: Lessons From Two States. Acad. Pediatr. 2015, 15, 430–438. [Google Scholar] [CrossRef] [PubMed]
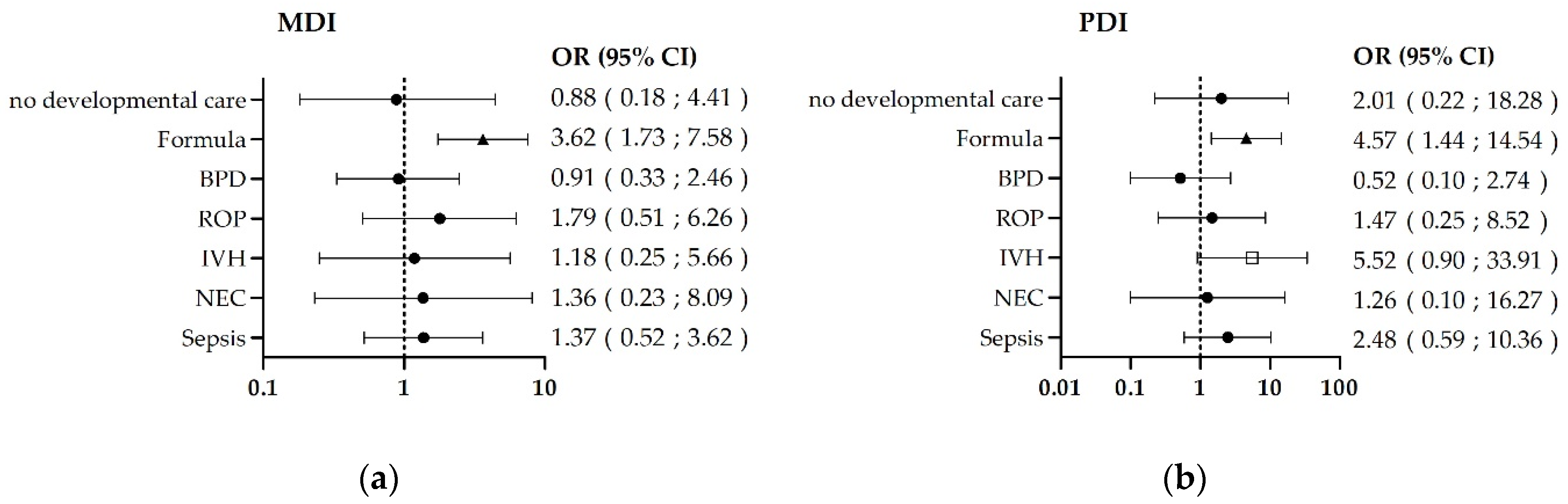
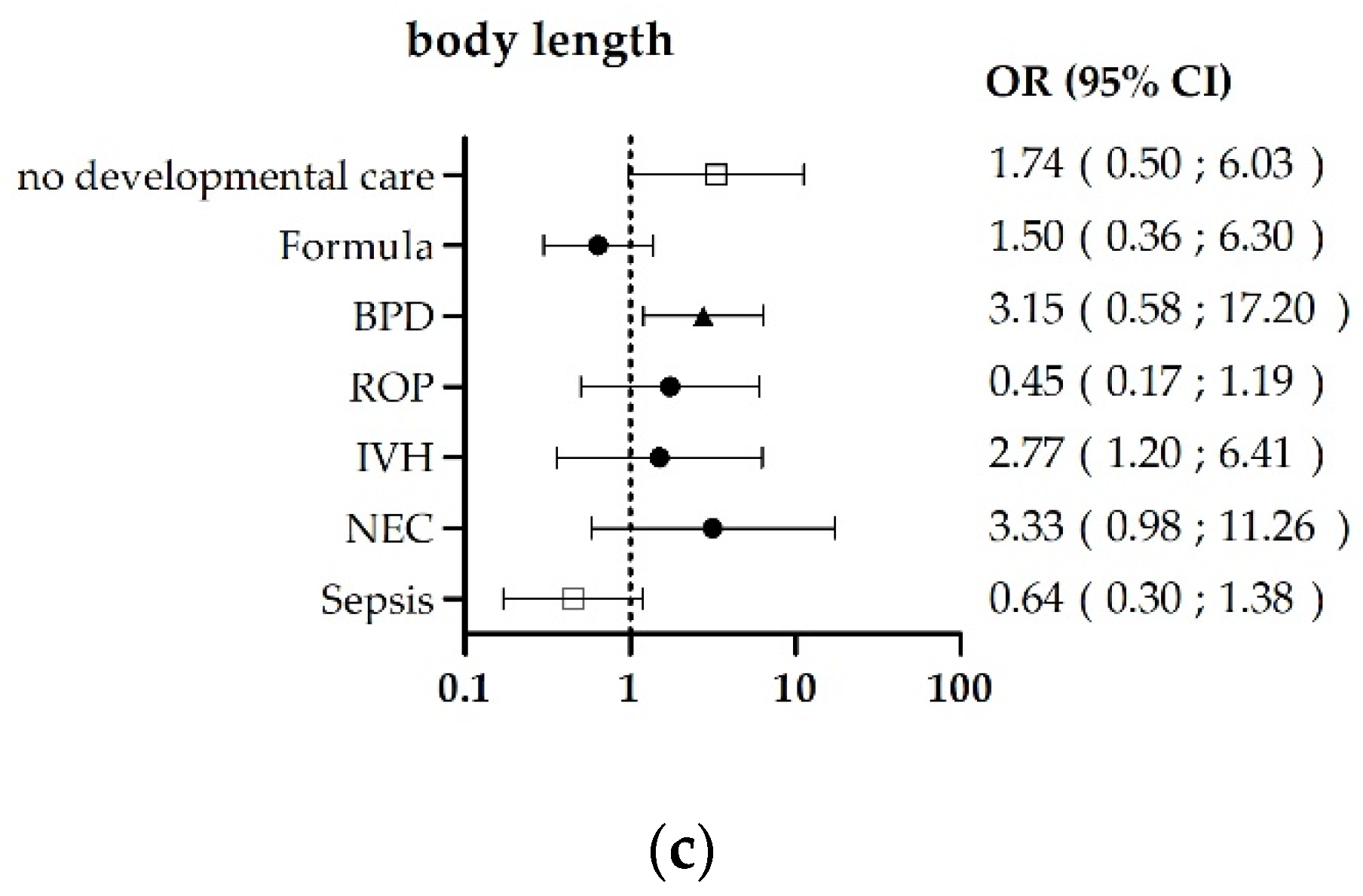
| n | % | ||
|---|---|---|---|
| Female | 129 | 50 | |
| gestational age at birth (weeks) | mv ± sd | 29 ± 2 | |
| <24 | 3 | 1 | |
| 24–27 + 6 | 77 | 30 | |
| 28–31 + 6 | 157 | 61 | |
| ≥32 | 19 | 7 | |
| birth weight (g) | mv ± sd | 1187 ± 347 | |
| <750 | 30 | 12 | |
| 750–999 | 59 | 23 | |
| 1000–1499 | 127 | 50 | |
| ≥1500 | 40 | 16 | |
| head circumference at birth (cm) | mv ± sd | 27.0 ± 3.1 | |
| risk factors | |||
| exclusively formula until 6 months age | 63 | 26 | |
| sepsis until discharge | 40 | 16 | |
| BPD until discharge | 43 | 17 | |
| IVH until discharge | |||
| grade 1 | 20 | 8 | |
| grade 2 | 7 | 3 | |
| grade 3 und 4 | 7 | 3 | |
| NEC until discharge | 8 | 3 | |
| ROP until discharge | 61 | 24 | |
| stage 1 | 44 | 17 | |
| stage 2 | 11 | 4 | |
| stage 3 | 6 | 3 | |
| <85/<10. Perc | ≤70/≤3. Perc | Total | |||
|---|---|---|---|---|---|
| n | % | n | % | mv ± sd | |
| All (n, % = 256, 100%) | |||||
| MDI | 37 | 15 | 7 | 3 | 97 ± 15 |
| PDI | 12 | 5 | 11 | 4 | 101 ± 13 |
| Body weight (g) | 32 | 13 | 57 | 23 | 11,354 ± 1693 |
| Body length (cm) | 26 | 10 | 45 | 18 | 86 ± 5 |
| Head circumference (cm) | 47 | 19 | 43 | 17 | 48 ± 2 |
| Formula (n, % = 63/27%) | |||||
| MDI | 23 | 37 | 6 | 10 | 90 ± 15 |
| PDI | 8 | 13 | 4 | 7 | 95 ± 13 |
| Body weight (g) | 22 | 36 | 12 | 19 | 11,300 ± 1606 |
| Body length (cm) | 14 | 23 | 6 | 10 | 87 ± 4 |
| Head circumference (cm) | 26 | 37 | 10 | 16 | 48 ± 2 |
| Sepsis (n, % = 40, 16%) | |||||
| MDI | 10 | 25 | 3 | 8 | 95 ± 16 |
| PDI | 6 | 15 | 3 | 8 | 99 ± 17 |
| Body weight (g) | 13 | 33 | 8 | 21 | 10,900 ± 1301 |
| Body length (cm) | 10 | 26 | 7 | 18 | 85 ± 4 |
| Head circumference (cm) | 18 | 46 | 8 | 21 | 47 ± 2 |
| BPD (n, % = 43, 17%) | |||||
| MDI | 10 | 23 | 2 | 5 | 94 ± 15 |
| PDI | 5 | 12 | 2 | 5 | 98 ± 17 |
| Body weight (g) | 21 | 50 | 16 | 38 | 10,554 ± 1350 |
| Body length (cm) | 20 | 48 | 14 | 33 | 84 ± 4 |
| Head circumference (cm) | 24 | 57 | 9 | 21 | 47 ± 2 |
| IVH ≥ grade 2 (n, % = 14, 5%) | |||||
| MDI | 4 | 29 | 2 | 14 | 88 ± 19 |
| PDI | 3 | 21 | 2 | 14 | 91 ± 16 |
| Body weight (g) | 9 | 70 | 6 | 46 | 9870 ± 1229 |
| Body length (cm) | 6 | 46 | 5 | 38 | 83 ± 3 |
| Head circumference (cm) | 7 | 54 | 4 | 31 | 47 ± 2 |
| NEC (n, % = 8, 3%) | |||||
| MDI | 3 | 38 | 1 | 13 | 89 ± 18 |
| PDI | 2 | 25 | 1 | 13 | 92 ± 20 |
| Body weight (g) | 4 | 57 | 2 | 29 | 11,231 ± 1778 |
| Body length (cm) | 4 | 57 | 3 | 43 | 83 ± 4 |
| Head circumference (cm) | 1 | 14 | 1 | 14 | 48 ± 1 |
| ROP ≥ stage 2 (n, % = 17, 7%) | |||||
| MDI | 6 | 35 | 2 | 12 | 92 ± 18 |
| PDI | 3 | 18 | 1 | 6 | 94 ± 14 |
| Body weight (g) | 10 | 59 | 5 | 29 | 10,435 ± 1504 |
| Body length (cm) | 8 | 47 | 5 | 29 | 83 ± 4 |
| Head circumference (cm) | 10 | 59 | 6 | 35 | 46 ± 2 |
| BPD | Developmental Care | OR (95% CI) | p-Value | ||
|---|---|---|---|---|---|
| Without (n = 2) | With (n = 37) | ||||
| MDI | mv ± sd | 96 ± 14 | 94 ± 15 | <0.10 * | † |
| PDI | 109 ± 6 | 98 ± 17 | <0.10 * | ||
| Body weight (g) | 10,450 ± 2758 | 10,679 ± 1235 | 1.21 (0.06; 23.24) | ||
| Body length (cm) | 84.3 ± 7.4 | 84.6 ± 3.9 | 1.23 (0.06; 23.90) | ||
| Head circumference (cm) | 47.3 ± 0.4 | 46.9 ± 1.4 | >100 * | ||
| Formula feeding | Developmental care | OR (95% CI) | p-value | ||
| Without (n = 3) | With (n = 54) | ||||
| MDI | mv ± sd | 83 ± 10 | 90 ± 14 | 1.40 (0.10; 18.86) | † |
| PDI | 94 ± 14 | 95 ± 13 | >100 * | ||
| Body weight (g) | 11,250 ± 1465 | 11,250 ± 1665 | 0.97 (0.06; 10.37) | ||
| Body length (cm) | 85.3 ± 4.3 | 86.4 ± 4.2 | 1.85 (0.14; 25.33) | ||
| Head circumference (cm) | 47.8 ± 0.7 | 47.5 ± 1.9 | 0.53 (0.03; 8.56) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lademann, H.; Janning, A.; Müller, J.; Neumann, L.; Olbertz, D.; Däbritz, J. Risk Factors of Growth Retardation and Developmental Deficits in Very Preterm Infants in a German Tertiary Neonatal Unit. Children 2021, 8, 394. https://doi.org/10.3390/children8050394
Lademann H, Janning A, Müller J, Neumann L, Olbertz D, Däbritz J. Risk Factors of Growth Retardation and Developmental Deficits in Very Preterm Infants in a German Tertiary Neonatal Unit. Children. 2021; 8(5):394. https://doi.org/10.3390/children8050394
Chicago/Turabian StyleLademann, Hanne, Anna Janning, Josephyn Müller, Luisa Neumann, Dirk Olbertz, and Jan Däbritz. 2021. "Risk Factors of Growth Retardation and Developmental Deficits in Very Preterm Infants in a German Tertiary Neonatal Unit" Children 8, no. 5: 394. https://doi.org/10.3390/children8050394
APA StyleLademann, H., Janning, A., Müller, J., Neumann, L., Olbertz, D., & Däbritz, J. (2021). Risk Factors of Growth Retardation and Developmental Deficits in Very Preterm Infants in a German Tertiary Neonatal Unit. Children, 8(5), 394. https://doi.org/10.3390/children8050394






