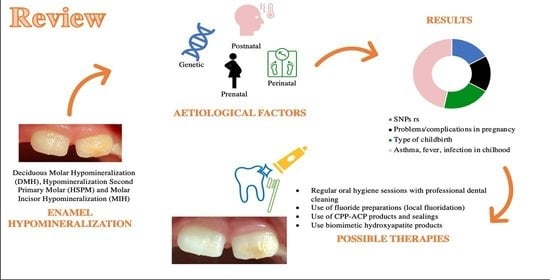
Assessment of Genetical, Pre, Peri and Post Natal Risk Factors of Deciduous Molar Hypomineralization (DMH), Hypomineralized Second Primary Molar (HSPM) and Molar Incisor Hypomineralization (MIH): A Narrative Review
Abstract
:1. Introduction
2. Material and Methods
2.1. Focused Question
2.2. Elegibility Criteria
2.3. Search Strategy
2.4. Research
2.5. Screening and Selection of Articles
2.6. Search Outcome and Evaluation
3. Results
3.1. Study Selection
3.2. Synthesis of Results
3.2.1. MIH (Sixteen Studies)
3.2.2. DMH (Two Studies)
3.2.3. HPSM (Two Studies)
3.2.4. MIH and DMH (One Study)
3.2.5. MIH and HPSM (Three Studies)
3.2.6. Genetic Factors
3.2.7. Pre-, Peri-, Post-Natal Factors
3.2.8. Results of Single Studies and Bias
4. Discussion
| Etiological Factors | Clinical Signs | Possible Therapy |
|---|---|---|
|
|
|
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berkovitz, B.; Holland, G.; Moxham, B. Oral Anatomy, Histology and Embriology, 5th ed.; Elsevier: New York, NY, USA, 2017. [Google Scholar]
- Chen, H.; Liu, Y. Advanced Ceramics for Dentistry; Butterworth-Heinemann: Oxford, UK, 2014; pp. 5–21. [Google Scholar]
- da Cunha Coelho, A.S.E.; Mata, P.C.M.; Lino, C.A.; Macho, V.M.P.; Areias, C.M.F.G.P.; Norton, A.P.M.A.P.; Augusto, A.P.C.M. Dental hypomineralization treatment: A systematic review. J. Esthet. Restor. Dent. 2019, 31, 26–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintero, Y.; Restrepo, M.; Saldarriaga, J.A.; Saldarriaga, A.; Santos-Pinto, L. Treatment options for deciduous molar hypomineralization: A report of three cases. Dent. Update 2019, 46, 546–553. [Google Scholar] [CrossRef]
- Elfrink, M.E.; Moll, H.A.; Kiefte-de Jong, J.C.; Jaddoe, V.W.; Hofman, A.; ten Cate, J.M.; Veerkamp, J.S. Pre- and postnatal determinants of deciduous molar hypomineralization in 6-year-old children. The generation R study. PLoS ONE 2014, 9, e91057. [Google Scholar] [CrossRef] [Green Version]
- Elfrink, M.E.; Schuller, A.A.; Weerheijm, K.L.; Veerkamp, J.S. Hypomineralized second primary molars: Prevalence and data in Dutch 5-year-olds. Carie Res. 2008, 42, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Elfrink, M.E.C.; Ghanim, A.; Manton, D.J.; Weerheijm, K.L. Standardized studies on Molar Incisor Hypomineralization (MIH) and Hypomineralized Second Primary Molars (HSPM): A need. Eur. Arch. Paediatr. Dent. 2015, 16, 247–255. [Google Scholar] [CrossRef]
- Weerheijm, K.L.; Jälevik, B.; Alaluusua, S. Molar-incisor hypomineralisation. Caries Res. 2001, 35, 390–391. [Google Scholar] [CrossRef]
- Farah, R.A.; Monk, B.C.; Swain, M.V.; Drummond, B.K. Protein content of molar- incisor hypomineralization enamel. J. Dent. 2010, 38, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Jeremias, F.; Koruyucu, M.; Küchler, E.C.; Bayram, M.; Tuna, E.B.; Deeley, K.; Pierri, R.A.; Souza, J.F.; Fragelli, C.M.; Paschoal, M.A.; et al. Genes expressed in dental enamel development are associated with molar-incisor hypomineralization. Arch. Oral Biol. 2013, 58, 1434–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerreth, K.; Zaorska, K.; Zabel, M.; Nowicki, M.; Borysewicz-Lewicka, M. Significance of genetic variations in developmental enamel defects of primary dentition in Polish children. Clin. Oral Investig. 2018, 22, 321–329. [Google Scholar] [CrossRef] [Green Version]
- Alaluusua, S. Aetiology of molar-incisor hypomineralization: A systematic review. Eur. Arch. Paediatr. Dent. 2010, 11, 53–58. [Google Scholar] [CrossRef]
- Jeremias, F.; Pierri, R.A.; Souza, J.F.; Fragelli, C.M.; Restrepo, M.; Finoti, L.S.; Bussaneli, D.G.; Cordeiro, R.C.; Secolin, R.; Maurer-Morelli, C.V.; et al. Family-Based Genetic Association for Molar-Incisor Hypomineralization. Caries Res. 2016, 50, 310–318. [Google Scholar] [CrossRef] [Green Version]
- Koruyucu, M.; Özel, S.; Tuna, E.B. Prevalence and etiology of molar-incisor hypomineralization (MIH) in the city of Istanbul. J. Dent. Sci. 2018, 13, 318–328. [Google Scholar] [CrossRef]
- Rai, A.; Singh, A.; Menon, I.; Singh, J.; Rai, V.; Aswal, G.S. Molar Incisor Hypomineralization: Prevalence and Risk Factors among 7–9 Years Old School Children in Muradnagar, Ghaziabad. Open Dent. J. 2018, 12, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Tourino, L.F.; Corrêa-Faria, P.; Ferreira, R.C.; Bendo, C.B.; Zarzar, P.M.; Vale, M.P. Association between Molar Incisor Hypomineralization in Schoolchildren and Both Prenatal and Postnatal Factors: A Population-Based Study. PLoS ONE 2016, 11, e0156332. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.J.P.B.; Andrade, N.S.; Queiroz, L.C.C.; Mendes, F.M.; Moura, M.S.; Moura, L.F.A.D.; Lima, M.D.M. Exploring the association between genetic and environmental factors and molar incisor hypomineralization: Evidence from a twin study. Int. J. Paediatr. Dent. 2018, 28, 198–206. [Google Scholar] [CrossRef]
- Allazzam, S.M.; Alaki, S.M.; El Meligy, O.A. Molar incisor hypomineralization, prevalence, and etiology. Int. J. Dent. 2014, 2014, 234508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garot, E.; Manton, D.; Rouas, P. Peripartum events and molar-incisor hypomineraliatzion (MIH) amongst young patients in southwest France. Eur. Arch. Paediatr. Dent. 2016, 17, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Kühnisch, J.; Thiering, E.; Heitmüller, D.; Tiesler, C.M.; Grallert, H.; Heinrich-Weltzien, R.; Hickel, R.; Heinrich, J.; GINI-10 Plus Study Group; LISA-10Plus Study Group. Genome-wide association study (GWAS) for molar-incisor hypomineralization (MIH). Clin. Oral Investig. 2014, 18, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Mejía, J.D.; Restrepo, M.; González, S.; Álvarez, L.G.; Santos-Pinto, L.; Escobar, A. Molar Incisor Hypomineralization in Colombia: Prevalence, Severity and Associated Risk Factors. J. Clin. Pediatric Dent. 2019, 43, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Giuca, M.R.; Cappè, M.; Carli, E.; Lardani, L.; Pasini, M. Investigation of Clinical Characteristics and Etiological Factors in Children with Molar Incisor Hypomineralization. Int. J. Dent. 2018, 2018, 7584736. [Google Scholar] [CrossRef] [PubMed]
- Elzein, R.; Chouery, E.; Abdel-Sater, F.; Bacho, R.; Ayoub, F. Molar-incisor hypomineralization in Lebanon: Association with prenatal, natal and postnatal factors. Eur. Arch. Paediatr. Dent. 2020, 22, 283–290. [Google Scholar] [CrossRef]
- Sönmez, H.; Yıldırım, G.; Bezgin, T. Putative factors associated with molar incisor hypomineralization: An epidemiological study. Eur. Arch. Paediatr. Dent. 2013, 14, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Hočevar, L.; Kovač, J.; Podkrajšek, K.T.; Battelino, S.; Pavlič, A. The possible influence of genetic etiological factors on molar-incisor hypomineralization. Arch. Oral Biol. 2020, 118, 104848. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.F.; Jeremias, F.; Costa-Silva, C.M.; Santos-Pinto, L.; Zuanon, A.C.; Cordeiro, R.C. Aetiology of molar-incisor hypomineralization (MIH) in Brazilian children. Eur. Arch. Paediatr. Dent. 2013. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, A.; Manton, D.; Bailey, D.; Mariño, R.; Morgan, M. Risk factors in the occurrence of molar-incisor hypomineralization amongst a group of Iraqi children. Int. J. Paediatr. Dent. 2013, 23, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Bezamat, M.; Souza, J.F.; Silva, F.M.F.; Corrêa, E.G.; Fatturi, A.L.; Brancher, J.A.; Carvalho, F.M.; Cavallari, T.; Bertolazo, L.; Machado-Souza, C.; et al. Gene-environment interaction in molar-incisor hypomineralization. PLoS ONE 2021, 16, e0241898. [Google Scholar] [CrossRef] [PubMed]
- Elfrink, M.E.; Moll, H.A.; Kiefte-de Jong, J.C.; El Marroun, H.; Jaddoe, V.W.; Hofman, A.; Stricker, B.H.; ten Cate, J.M.; Veerkamp, J.S. Is maternal use of medicines during pregnancy associated with deciduous molar hypomineralization in the offspring? A prospective, population-based study. Drug Saf. 2013, 36, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.R.S.; Pereira, A.S.; de Moura, M.S.; Lima, C.C.B.; Paiva, S.M.; Moura, L.F.A.D.; de Deus Moura de Lima, M. Pre-term birth and asthma is associated with hypomineralized second primary molars in pre-schoolers: A population-based study. Int. J. Paediatr. Dent. 2020, 30, 193–201. [Google Scholar] [CrossRef]
- Silva, M.J.; Kilpatrick, N.M.; Craig, J.M.; Manton, D.J.; Leong, P.; Burgner, D.; Scurrah, K.J. Etiology of Hypomineralized Second Primary Molars: A Prospective Twin Study. J. Dent. Res. 2019, 98, 77–83. [Google Scholar] [CrossRef]
- Costa-Silva, C.M.; Simpson de Paula, J.; Ambrosano, G.M.B.; Mialhe, F.L. Influence of deciduous molar hypomineralization on the development of molar-incisor hypomineralizarion. Braz. J. Oral Sci. 2013, 12, 335–338. [Google Scholar] [CrossRef]
- Fatturi, A.L.; Menoncin, B.L.; Reyes, M.T.; Meger, M.; Scariot, R.; Brancher, J.A.; Küchler, E.C.; Feltrin-Souza, J. The relationship between molar incisor hypomineralization, dental caries, socioeconomic factors, and polymorphisms in the vitamin D receptor gene: A population-based study. Clin. Oral Investig. 2020, 24, 3971–3980. [Google Scholar] [CrossRef] [PubMed]
- van der Tas, J.T.; Elfrink, M.E.C.; Heijboer, A.C.; Rivadeneira, F.; Jaddoe, V.W.V.; Tiemeier, H.; Schoufour, J.D.; Moll, H.A.; Ongkosuwito, E.M.; Wolvius, E.B.; et al. Foetal, neonatal and child vitamin D status and enamel hypomineralization. Community Dent. Oral Epidemiol. 2018, 46, 343–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Tas, J.T.; Elfrink, M.E.; Vucic, S.; Heppe, D.H.; Veerkamp, J.S.; Jaddoe, V.W.; Rivadeneira, F.; Hofman, A.; Moll, H.A.; Wolvius, E.B. Association between Bone Mass and Dental Hypomineralization. J. Dent. Res. 2016, 95, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Crombie, F.; Manton, D.; Kilpatrick, N. Aetiology of molar-incisor hypomineralization: A critical review. Int. J. Paediatr. Dent. 2009, 19, 73–83. [Google Scholar] [CrossRef]
- Stephanopoulos, G.; Garefalaki, M.E.; Lyroudia, K. Genes and related proteins involved in amelogenesisimperfecta. J. Dent. Res. 2005, 84, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.C.; Estrella, N.M.; Milkovich, R.N.; Kim, J.W.; Simmer, J.P.; Hu, J.C. Target gene analyses of 39 amelogenesis imperfecta kindreds. Eur. J. Oral Sci. 2011, 119, 311–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasaki, K.; Bajenova, E.; Somogyi-Ganss, E.; Miller, M.; Nguyen, V.; Nourkeyhani, H.; Gao, Y.; Wendel, M.; Ganss, B. Amelotin—A novel secreted, ameloblast-specific protein. J. Dent. Res. 2005, 84, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Scurrah, K.J.; Craig, J.M.; Manton, D.J.; Kilpatrick, N. Etiology of molar incisor hypomineralization—A systematic review. Community Dent. Oral Epidemiol. 2016, 44, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Giuca, M.R.; Lardani, L.; Pasini, M.; Beretta, M.; Gallusi, G.; Campanella, V. State-of-the-art on MIH. Part. 1 Definition and aepidemiology. Eur. J. Paediatr. Dent. 2020, 21, 80–82. [Google Scholar]
- Beentjes, V.E.; Weerheijm, K.L.; Groen, H.J. Factors involved in the aetiology of molar-incisor hypomineralisation (MIH). Eur. J. Paediatr. Dent. 2002, 3, 9–13. [Google Scholar]
- Elhennawy, K.; Schwendicke, F. Managing molar-incisor hypomineralization: A systematic review. J. Dent. 2016, 55, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Fütterer, J.; Ebel, M.; Bekes, K.; Klode, C.; Hirsch, C. Influence of customized therapy for molar incisor hypomineralization on children’s oral hygiene and quality of life. Clin. Exp. Dent. Res. 2020, 6, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, J.; Albadri, S. Management of Molar Incisor Hypomineralization (MIH): A 1-Year Retrospective Study in a Specialist Secondary Care Centre in the UK. Children 2020, 7, 252. [Google Scholar] [CrossRef] [PubMed]
- Scribante, A.; Dermenaki Farahani, M.R.; Marino, G.; Matera, C.; Rodriguez YBaena, R.; Lanteri, V.; Butera, A. Biomimetic Effect of Nano-Hydroxyapatite in Demineralized Enamel before Orthodontic Bonding of Brackets and Attachments: Visual, Adhesion Strength, and Hardness in In Vitro Tests. BioMed Res. Int. 2020, 2020, 6747498. [Google Scholar] [CrossRef] [Green Version]
- Murri Dello Diago, A.; Cadenaro, M.; Ricchiuto, R.; Banchelli, F.; Spinas, E.; Checchi, V.; Giannetti, L. Hypersensitivity in Molar Incisor Hypomineralization: Superficial Infiltration Treatment. Appl. Sci. 2021, 11, 1823. [Google Scholar] [CrossRef]
- Elhussein, M.; Jamal, H. Molar Incisor Hypomineralization-To Extract or to Restore beyond the Optimal Age? Children 2020, 7, 91. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Morittu, S.; Barbieri, S.; Bruni, A.; Sinesi, A.; Ricci, M.; Trombini, J.; et al. Assessment of Genetical, Pre, Peri and Post Natal Risk Factors of Deciduous Molar Hypomineralization (DMH), Hypomineralized Second Primary Molar (HSPM) and Molar Incisor Hypomineralization (MIH): A Narrative Review. Children 2021, 8, 432. https://doi.org/10.3390/children8060432
Butera A, Maiorani C, Morandini A, Simonini M, Morittu S, Barbieri S, Bruni A, Sinesi A, Ricci M, Trombini J, et al. Assessment of Genetical, Pre, Peri and Post Natal Risk Factors of Deciduous Molar Hypomineralization (DMH), Hypomineralized Second Primary Molar (HSPM) and Molar Incisor Hypomineralization (MIH): A Narrative Review. Children. 2021; 8(6):432. https://doi.org/10.3390/children8060432
Chicago/Turabian StyleButera, Andrea, Carolina Maiorani, Annalaura Morandini, Manuela Simonini, Stefania Morittu, Stefania Barbieri, Ambra Bruni, Antonia Sinesi, Maria Ricci, Julia Trombini, and et al. 2021. "Assessment of Genetical, Pre, Peri and Post Natal Risk Factors of Deciduous Molar Hypomineralization (DMH), Hypomineralized Second Primary Molar (HSPM) and Molar Incisor Hypomineralization (MIH): A Narrative Review" Children 8, no. 6: 432. https://doi.org/10.3390/children8060432
APA StyleButera, A., Maiorani, C., Morandini, A., Simonini, M., Morittu, S., Barbieri, S., Bruni, A., Sinesi, A., Ricci, M., Trombini, J., Aina, E., Piloni, D., Fusaro, B., Colnaghi, A., Pepe, E., Cimarossa, R., & Scribante, A. (2021). Assessment of Genetical, Pre, Peri and Post Natal Risk Factors of Deciduous Molar Hypomineralization (DMH), Hypomineralized Second Primary Molar (HSPM) and Molar Incisor Hypomineralization (MIH): A Narrative Review. Children, 8(6), 432. https://doi.org/10.3390/children8060432