Deformity Reconstruction Surgery for Tibial Hemimelia
Abstract
:1. Introduction
2. Evaluation
3. Associations
4. Classification
5. Treatment Options
5.1. Reconstruction
5.2. Amputation
5.3. Limb Lengthening
6. Author’s Type-Specific Reconstruction
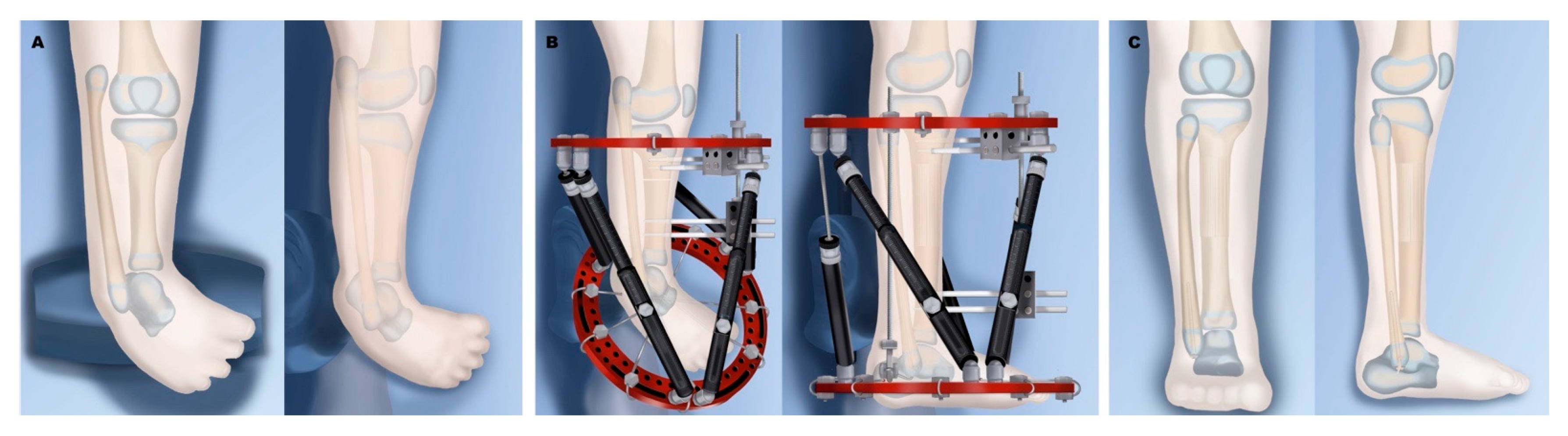
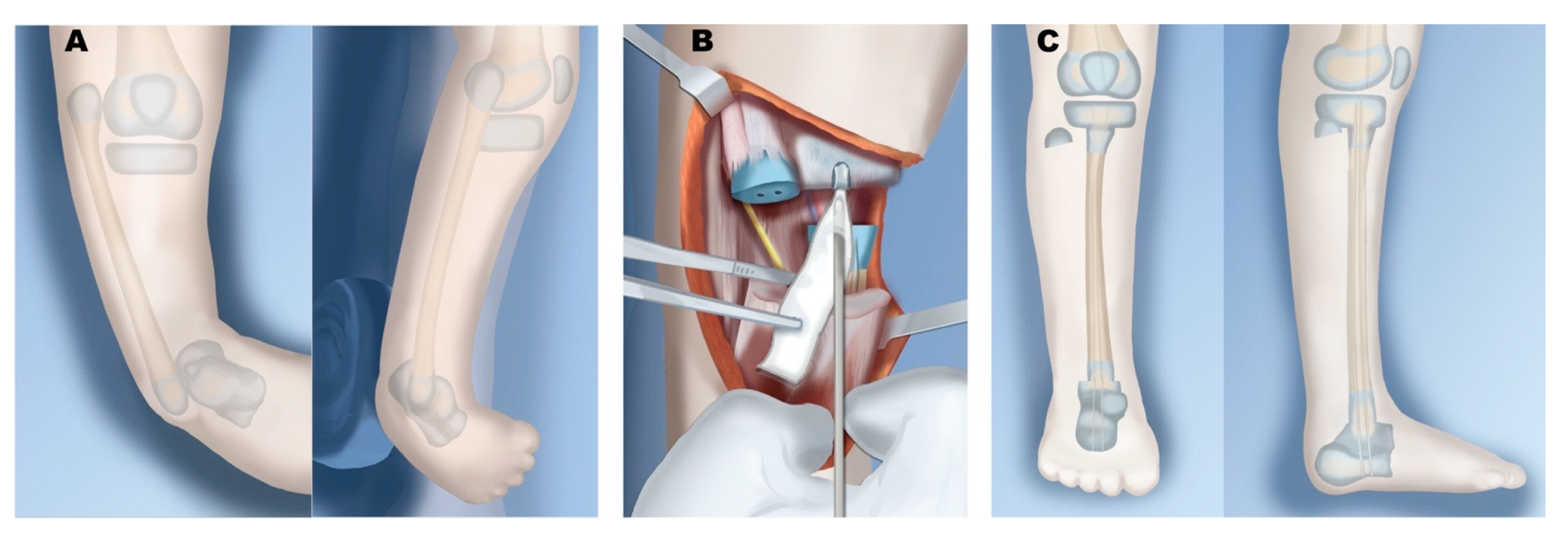
6.1. Paley Type 1
6.2. Paley Type 2
6.3. Paley Type 3
6.4. Paley Type 4
6.5. Paley Type 5
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, F.W. The Brown Operation for Total Hemimelia Tibia. In Selected Lower-Limb Anomalies: Surgical and Prosthetics Management: A Symposium Held in Washington; Aitken, G.T., Ed.; The National Academies Press: Washington, DC, USA, 1971. [Google Scholar]
- Weber, M.; Schroder, S.; Berdel, P.; Niethard, F.U. Nation-wide registration of limb deficiencies in Germany. Z. Orthop. Ihre. Grenzgeb. 2005, 143, 534–538. [Google Scholar] [CrossRef]
- Otto, A.W. Monstrorum sexcentorum descriptio anatomica. Vratislaviae (Breslau): Sumptibus Ferdinandi Hirt, 1841. Print.
- Billroth, T. Ueber einige durch Knochendefecte bedingte Krümmungen des Fusses. Arch Klin. Chir. 1861, 1, 252–268. [Google Scholar]
- Dankmeijer, J. Congenital absence of the tibia. Anat. Rec. 1935, 62, 179194. [Google Scholar] [CrossRef]
- Nutt, J.; Smith, E. Total congenital absence of the tibia. Am. J. Roentgen 1941, 46, 841. [Google Scholar]
- Kaplan-List, K.; Klionsky, N.; Sanders, J.O.; Katz, M.E. Systematic radiographic evaluation of tibial hemimelia with orthopedic implications. Pediatric Radiol. 2017, 47, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Turker, R.; Mendelson, S.; Ackman, J.; Lucicky, J.P. Anatomic considerations of the foot and leg in tibial hemimelia. J. Pediatric Orthop. 1996, 16, 445–449. [Google Scholar] [CrossRef]
- Granite, G.; Herzenberg, J.E.; Wade, R. Rare case of tibial hemimelia, preaxial polydactyly, and club foot. World J. Clin. Cases 2016, 4, 401. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.; Hecht, J.T.; Taylor, S.; Wilkins, I. Tibial hemimelia syndrome: Prenatal diagnosis by real-time ultrasound. Prenat. Diagn. 1994, 14, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Aitken, G.T.; Bose, K.; Brown, F.W. Tibial Hemimelia. In Campbell’s Operative Orthopaedics; Mosby-Year Book: St. Louis, MO, USA, 1998. [Google Scholar]
- Spiegel, D.; Loder, R.; Crandall, R. Congenital longitudinal deficiency of the tibia. Int. Orthop. 2003, 27, 338–342. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.; Barnes, J.; Lloyd-Roberts, G. Congenital aplasia and dysplasia of the tibia with intact fibula. Classification and management. J. Bone Jt. Surg. Br. Vol. 1978, 60, 31–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aitken, G.T. Tibial Hemimelia, in Selected Lower-Limb Anomalies: Surgical and Prosthetics Management: A Symposium Held in Washington; The National Academies Press: Washington, DC, USA, 1971; pp. 1–19. [Google Scholar]
- Emami-Ahari, Z. Bilateral absence of the tibias in three sibs. Birth Defects Orig. Art. Ser. 1974, 1974 10, 197–200. [Google Scholar]
- Clark, M.W. Autosomal dominant inheritance of tibial meromelia. Report of a kindred. J. Bone and Jt. Surg. Am. Vol. 1975, 57, 262–264. [Google Scholar] [CrossRef]
- Lenz, W. Genetics and limb deficiencies. Clin. Orthop. Relat. Res. 1980, 148, 9–17. [Google Scholar] [CrossRef]
- Fried, K.; Goldberg, M.D.; Mundel, G.; Reif, R. Severe lower limb malformation associated with other deformities and death in infancy in two brothers. J. Med. Genet. 1977, 14, 352–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahloudji, M.; Farpour, H. An unusual limb deformity in an inbred community. Birth Defects Orig. Artic. Series 1974, 10, 75–80. [Google Scholar]
- McKay, M.; Clarren, S.K.; Zorn, R.; Opitz, J.M. Isolated tibial hemimelia in sibs: An autosomal-recessive disorder? Am. J. Med. Genet. 1984, 17, 603–607. [Google Scholar] [CrossRef]
- Taleb, H.; Afshar, A.; Abdi Rad, I.; Tabrizi, A.; Ghazani, R.B.; Bateni, A. A High Prevalence Rate of Tibia Hemimelia in a Subregion of West Azarbaijan, Iran. J. Pediatr. Genet. 2019, 8, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.L.; Smith, N.R. Congenital absence of tibia. Arch. Dis. Child. 1926, 1, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hovelacque, A.; Noel, R. Processus embryologique de l’absence congenitale du tibia. C. R. Soc. Biol. Paris 1923, 88, 577–578. [Google Scholar]
- Leite, J.A.D.; Lima, L.C.; Sampaio, M.L.B. Tibial hemimelia in one of the identical twins. J. Pediatric Orthop. 2010, 30, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Werner, P. Ueber einen seltenen Fall von Zwergwuchs. Arch. Gynaekologie 1915, 104, 278–300. [Google Scholar] [CrossRef]
- Vargas, F.R.; Pontes, R.L.; Llerena Jr, J.C.; de Almedia, J.C.C. Absent tibiae—polydactyly—triphalangeal thumbs with fibular dimelia: Variable expression of the Werner (McKusick 188770) syndrome? Am. J. Med. Genet. 1995, 55, 261–264. [Google Scholar] [CrossRef]
- Stevens, C.A.; Moore, C.A. Tibial hemimelia in Langer-Giedion syndrome—possible gene location for tibial hemimelia at 8q. Am. J. Med. Genet. 1999, 85, 409–412. [Google Scholar] [CrossRef]
- Habou, O.; Magagi, I.A.; Adamou, H. Gollop–Wolfgang Complex. J. Neonatal Surg. 2017 6, 19. [CrossRef]
- Prasad, C.; Quackenbush, E.J.; Whiteman, D.; Korf, B. Limb anomalies in DiGeorge and CHARGE syndromes. Am. J. Med. Genet. 1997, 68, 179–181. [Google Scholar] [CrossRef]
- Alazami, A.M.; Mzahrani, F.; Alkuraya, F.S. Expanding the E in CHARGE. Am. J. Med. Genet. Part A 2008, 146, 1890–1892. [Google Scholar] [CrossRef] [PubMed]
- Sanlaville, D.; Etchevers, H.C.; Gonzales, M.; Martinovic, J.; Clement-Ziza, M.; Delezoide, A.; Aubry, M.; Pelet, A.; Chemouny, S.; Cruaud, C. Phenotypic spectrum of CHARGE syndrome in fetuses with CHD7 truncating mutations correlates with expression during human development. J. Med. Genet. 2006, 43, 211–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, P.; Ma, A.; Wilson, M.; Williams, G.; Curotta, J.; Munns, C.F.; Mehr, S. CHARGE syndrome: A review. J. Paediatr. Child Health 2014, 50, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Schoenecker, P.L.; Capelli, A.M.; Millar, E.A.; Sheen, M.R.; Haher, T.; Aiona, M.D.; Meyer, L.C. Congenital longitudinal deficiency of the tibia. J. Bone Jt. Surg. Am. 1989, 71, 278–287. [Google Scholar] [CrossRef]
- Launois, P.a.K.G. Rev D’Orthop., Paris, 1901, pp. 327–411. (Referred to in Evans E.L., Smith N.R. Congenital Absence of Tibia. Arch. Dis. Child.) 1926, 1, 194–229.
- Chinnakkannan, S.; Das, R.R.; Rughmini, K.; Ahmed, S. A case of bilateral tibial hemimelia type VIIa. Indian J. Hum. Genet. 2013, 19, 108. [Google Scholar] [PubMed] [Green Version]
- Jose, R.M.; Kamath, A.K.; Vijayaraghavan, S.; Varghese, S.; Nair, S.R.; Nanadakumar, U.R. Tibial hemimelia with ‘mirror foot’. Eur. J. Plast. Surg. 2004, 27, 39–41. [Google Scholar]
- Yetkin, H.; Cila, E.; Guzel, V.B.; Kanatly, U. Femoral Bifurcation Associated with Tibial Hemimelia; SLACK Incorporated: Thorofare, NJ, USA, 2001. [Google Scholar]
- Orimolade, E.A.; Ikem, I.C.; Oginni, L.M.; Odunsi, A.O. Femoral bifurcation with ipsilateral tibia hemimelia: Early outcome of ablation and prosthetic fitting. Niger. J. Clin. Pract. 2011, 14, 492–494. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Palazzi, F.; Bendahan, J.; Rivas, S. Congenital deficiency of the tibia: A report on 22 cases. J. Pediatric Orthop. Part B 1998, 7, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Majewski, F.; Kuster, W.; Ter Haar, B.; Goecke, T. Aplasia of tibia with split–hand/split–foot deformity. Report of six families with 35 cases and consideration about variability and penetrance. Hum. Genet. 1985, 70, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Laurin, C.A.; Favreau, J.; Labelle, P. Bilateral absence of the radius and tibia with bilateral reduplication of the ulna and fibula: A case report. J. Bone Jt. Surg. Am. 1964, 46, 137–142. [Google Scholar] [CrossRef]
- Wiedemann, H.R.; Opitz, J.M. Unilateral partial tibia defect with preaxial polydactyly, general micromelia, and trigonomacrocephaly with a note on “developmental resistance”. Am. J. Med. Genet. 1983, 14, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Ondari, J.; Kinyanjui, J.; Miano, P.; Sang, E.; Oburu, E.; Maru, M. Femoral bifurcation and bilateral tibial hemimelia: Case report. Pan Afr. Med. J. 2018, 30, 99. [Google Scholar] [CrossRef] [PubMed]
- Salinas–Torres, V.M.; Barajas-Barajas, L.O.; Perez-Garcia, N.; Perez-Garcia, G. Bilateral tibial hemimelia type 1 (1a and 1b) with T9 and T10 hemivertebrae: A novel association. Sao Paulo Med. J. 2013, 131, 275–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bade, P. Zur Pathologie und Therapie des Tibiadefektes. Z. Orthop. Chir. 1906, 16, 150–166. [Google Scholar]
- Sulamaa, M.; Ryoeppy, S. Congenital absence of the tibia. Acta Orthop. Scand. 1964, 34, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Doi, K.; Baliarsing, A.S. Transtibial amputation with plantar flap for congenital deficiency of the tibia. Clin. Orthop. Relat. Res. 2002, 403, 186–190. [Google Scholar] [CrossRef]
- Frantz, C.H.; O’Rahilly, R. Congenital skeletal limb deficiencies. J. Bone Jt. Surg. Am. 1961, 43, 1202–1224. [Google Scholar] [CrossRef] [Green Version]
- Clinton, R.; Birch, J.G. Congenital tibial deficiency: A 37-year experience at 1 institution. J. Pediatric Orthop. 2015, 35, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Weber, M. New classification and score for tibial hemimelia. J. Child. Orthop. 2008, 2, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Devitt, A.T.; O’Donnell, T.; Fogarty, E.E.; Dowling, F.E.; Moore, D.P. Tibial hemimelia of a different class. J. Pediatric Orthop. 2000, 20, 616–622. [Google Scholar] [CrossRef]
- Paley, D.; Gillespie, R.H.J. Limb deficiency. In Pediatric Orthopaedic Secrets; Staheli, L.T., Ed.; Hanley & Belfus: Philadelphia, PA, USA, 2003; pp. 406–416. [Google Scholar]
- Paley, D. Tibial hemimelia. In Pediatric Lower Limb Deformities: Principles and Techniques of Management; Sabharwal, S., Ed.; Springer: Cham, Switzerland, 2015; pp. 455–481. [Google Scholar]
- Paley, D. Tibial hemimelia: New classification and reconstructive options. J. Child. Orthop. 2016, 10, 529–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrivastava, S.; Nawghare, S.; Dulani, R.; Singh, P.; Jain, S. A rare variant of tibial hemimelia and its treatment. J. Pediatric Orthop. B 2009, 18, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Shah, H. Tibial hypoplasia with a bifid tibia: An unclassified tibial hemimelia. Case Rep. 2016, 2016, bcr2016216622. [Google Scholar] [CrossRef] [PubMed]
- Senthil, V.; Kottamttavide, I.V.; Shah, H. Unclassified tibial hemimelia. Case Rep. 2016, 2016, bcr2016215305. [Google Scholar] [CrossRef] [PubMed]
- Albert, E. Wein Med Presse, 1877, p. 111. (Referred to in Evans E.L., Smith N.R. Congenital Absence of Tibia. Arch Dis Child). 1926, 1, 194–229.
- Myers, H. Congenital absence of tibia: Transplantation of head of fibula: Arthrodesis at the ankle-joint. J. Bone Jt. Surg. Am. 1905, 2, 72–85. [Google Scholar]
- Nove–Josserand, G. Bull Soc Chir Lyon. Precis d’Orthop, Paris 1905, 440, 1899. IV: p. 259. (Referred to in Evans E.L., Smith N.R. Congenital Absence of Tibia. Arch. Dis. Child.) 1926, 1, 194–229.
- Fraser, J.; Robarts, H. Congenital deficiency of the radius and a homologous condition in the leg. Lancet 1914, 183, 1606–1608. [Google Scholar] [CrossRef]
- Putti, V. The treatment of congenital absence of the tibia or fibula. Chir. Org. Mov. 1929, 7, 513. [Google Scholar]
- Kalamchi, A.; Dawe, R.W. Congenital deficiency of the tibia. J. Bone Jt. Surg. Br. 1985, 67, 581–584. [Google Scholar] [CrossRef] [Green Version]
- Loder, R.T.; Herring, J.A. Fibular transfer for congenital absence of the tibia: A reassessment. J. Pediatric Orthop. 1987, 7, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Kumar Sahoo, P.; Sahu, M.M.; Prasad Das, S. Clinical spectrum of congenital tibial hemimelia in 35 limbs of 24 patients: A single center observational study from India. Eur. J. Med. Genet. 2019, 62, 103666. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, N.M.; Ghaly, N.A. Surgical treatment of type II congenital dysplasia of the tibia, Pan Arab. J. Orthop. Trauma 2004, 8, 129–134. [Google Scholar]
- Wehbé, M.A.; Weinstein, S.L.; Ponseti, I.V. Tibial agenesis. J. Pediatric Orthop. 1981, 1, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mir, S.; Sharma, V.; Dar, I. Congenital Absence of the Tibia. JK Sci. 2002, 4, 213–214. [Google Scholar]
- Christini, D.; Levy, E.J.; Facanha, F.A.; Kumar, S.J. Fibular transfer for congenital absence of the tibia. J. Pediatric Orthop. 1993, 13, 378–381. [Google Scholar] [CrossRef]
- Shahcheraghi, G.H.; Javid, M. Functional assessment in tibial hemimelia (can we also save the foot in reconstruction?). J. Pediatric Orthop. 2016, 36, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Grissom, L.E.; Harcke, H.T.; Kumar, S.J. Sonography in the management of tibial hemimelia. Clin. Orthop. Relat. Res. 1990, 251, 266–270. [Google Scholar] [CrossRef]
- Brown, F.W. Construction of a knee joint in congenital total absence of the tibia (paraxial hemimelia tibia): A preliminary report. J. Bone Jt. Surg. Am. 1965, 47, 695–704. [Google Scholar] [CrossRef]
- Brown, F.; Pohnert, W. Construction of a knee joint in meromelia tibia (congenital absence of the tibia): A 15 year follow-up study. J. Bone Jt. Surg. Am. 1972, 54, 1333. [Google Scholar]
- Jayakumar, S.S.; Eilert, R.E. Fibular transfer for congenital absence of the tibia. Clin. Orthop. Relat. Res. 1979, 139, 97–101. [Google Scholar] [CrossRef]
- Epps, C.H., Jr.; Schneider, P. Treatment of hemimelias of the lower extremity. Long–term results. J. Bone Jt. Surg. Am. Vol. 1989, 71, 273–277. [Google Scholar] [CrossRef]
- Simmons, E.D., Jr.; Ginsburg, G.M.; Hall, J.E. Brown’s procedure for congenital absence of the tibia revisited. J. Pediatric Orthop. 1996, 16, 85–89. [Google Scholar] [CrossRef]
- Wada, A.; Nakamura, T.; Fujii, T.; Urano, N.; Yanagida, H.; Takamura, K.; Oketani, Y.; Kubota, H. Limb salvage treatment for Gollop–Wolfgang complex (femoral bifurcation, complete tibial hemimelia, and hand ectrodactyly). J. Pediatric Orthop. B 2013, 22, 457–463. [Google Scholar] [CrossRef]
- Hosny, G.A. Treatment of tibial hemimelia without amputation: Preliminary report. J. Pediatric Orthop. B 2005, 14, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Eamsobhana, P.; Kaewpornsawan, K. Limb salvage in tibial hemimelia. J. Med. Assoc. Thai. 2012, 95 (Suppl. S9), S62–S69. [Google Scholar]
- Laufer, A.; Frommer, A.; Gosheger, G.; Roedl, R.; Broeking, J.N.; Toporowski, G.; Rachbauer, A.M.; Vogt, B. Femoro-pedal distraction in staged reconstructive treatment of tibial aplasia. Bone Jt. J. 2020, 102, 1248–1255. [Google Scholar] [CrossRef]
- Courvoisier, A.; Sailhan, F.; Trevenin-Lemoine, C.; Vialle, R.; Damsin, J. Congenital tibial deficiencies: Treatment using the Ilizarov’s external fixator. Orthop. Traumatol. Surg. Res. 2009, 95, 431–436. [Google Scholar] [CrossRef] [Green Version]
- Tokmakova, K.; Riddle, E.C.; Kumar, S.J. Type IV congenital deficiency of the tibia. J. Pediatric Orthop. 2003, 23, 649–653. [Google Scholar] [CrossRef]
- Basso, M.; Camurri, V.; Frediani, P.; Boero, S. A rare case of tibial hemimelia, surgical technique and clinical results. Acta Orthop. Traumatol. Turc. 2018, 52, 315–319. [Google Scholar] [CrossRef]
- Weber, M. A new knee arthroplasty versus Brown procedure in congenital total absence of the tibia: A preliminary report. J. Pediatric Orthop. B 2002, 11, 53–59. [Google Scholar]
- Weber, M. Congenital Leg Deformities: Tibial Hemimelia, in Limb Lengthening and Reconstruction Surgery; Ilizarov, R., Ed.; Informa Healthcare USA Inc.: New York, NY, USA, 2007. [Google Scholar]
- Balcı, H.; Sağlam, Y.; Bilgili, F.; Şen, C.; Kocaoğlu, M.; Eralp, L. Preliminary report on amputation versus reconstruction in treatment of tibial hemimelia. Acta Orthop. Traumatol. Turc. 2015, 49, 627–633. [Google Scholar]
- De Sanctis, N.; Razzano, E.; Scognamiglio, R.; Rega, A.N. Tibial agenesis: A new rationale in management of type II-report of three cases with long–term follow–up. J. Pediatric Orthop. 1990, 10, 198–201. [Google Scholar] [CrossRef]
- Hootnick, D.; Boyd, N.A.; Fixsen, J.A.; Lloyd-Roberts, G.C. The natural history and management of congenital short tibia with dysplasia or absence of the fibula. J. Bone Jt. Surg. Br. Vol. 1977, 59, 267–271. [Google Scholar] [CrossRef] [Green Version]
- Brdar, R.; Petronic, I.; Abramovic, D.; Lukac, M.; Cirovic, D.; Knezevic, T.; Nikolic, D. Type III longitudinal deficiency of the tibia and outcome of reconstructive surgery in a female patient. Medicina 2010, 46, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onwuasoigwe, O. Longitudinal overgrowth of the femur stimulated by short–leg ambulation in unilateral partial tibia hemimelia. J. Pediatric Orthop. B 2013, 22, 357–362. [Google Scholar] [CrossRef]
- Javid, M.; Shahcheraghi, G.H.; Nooraie, H. Ilizarov lengthening in centralized fibulana. J. Pediatric Orthop. 2000, 20, 160–162. [Google Scholar] [CrossRef]
- Oetgen, M.E.; Richards, B.S. Complications associated with the use of bone morphogenetic protein in pediatric patients. J. Pediatric Orthop. 2010, 30, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishna, V.N.; Madhuri, V.; Palocaren, T. Optimizing the use of fibula in type II tibial hemimelia: Early results. J. Pediatric Orthop. B 2019, 28, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Wada, A.; Nakamura, T.; Urano, N.; Kubota, H.; Oketani, Y.; Taketa, M.; Fujii, T. Foot centralization for tibial hemimelia. J. Pediatric Orthop. B 2015, 24, 147–153. [Google Scholar] [CrossRef]
- Yadav, S.S. Type I Tibial Hemimelia: A Limb-Salvage and Lengthening Technique. JB JS Open Access 2019, 4, e0029. [Google Scholar] [CrossRef] [PubMed]
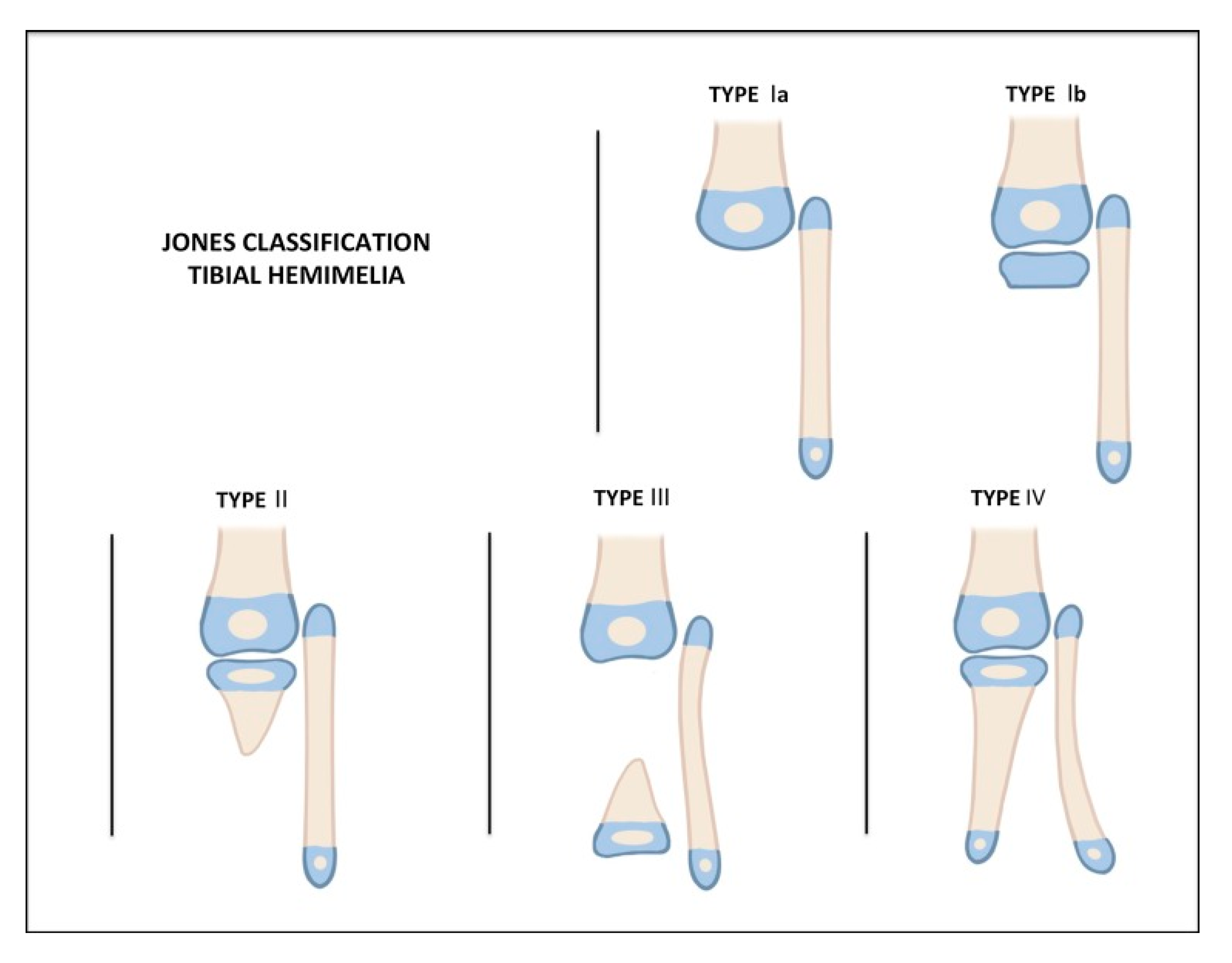
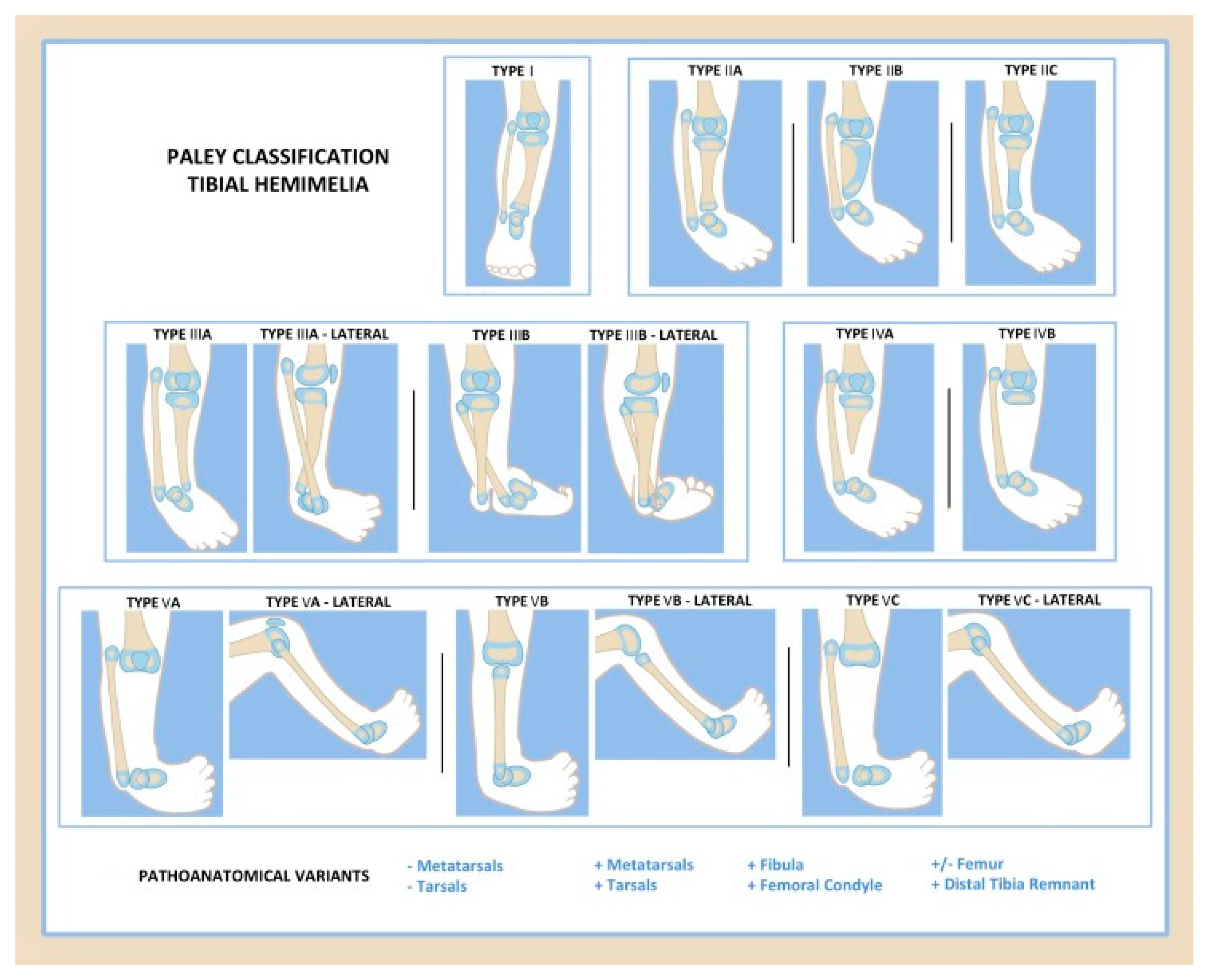







| Knee Joint | Proximal Tibia | Tibial Shaft | Distal Tibia | Ankle Joint/Foot | |
|---|---|---|---|---|---|
| Type 1 | Normal | Normal or valgus | Shortened | Normal | Normal |
| Type 2 | Normal | Normal or mild dysplasia (pagoda shaped) | Shortened | Variable: | Dysplastic; equinovarus foot |
| 2A | Well formed | ||||
| 2B | Delta tibia | ||||
| 2C | Cartilage anlage | ||||
| Type 3 | Normal | Normal | Varus/procurvatum | No plafond | Diastasis; equinovarus |
| 3A | Internally rotated around tibia | ||||
| 3B | Skin cleft, foot with fibula | ||||
| Type 4 | Normal | Present | Variable: | Absent | Equinovarus |
| 4A | Normal | Partial | |||
| 4B | Non-ossified/dysplastic | Absent | |||
| Type 5 | Flexion contracture | Complete aplasia | Absent | Absent | Equinovarus |
| 5A | (+)Quad (+)Patella | ||||
| 5B | (+)Quad (−)Patella | Autocentralized fibula | |||
| 5C | (−)Quad (−)Patella | No knee capsule |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chong, D.Y.; Paley, D. Deformity Reconstruction Surgery for Tibial Hemimelia. Children 2021, 8, 461. https://doi.org/10.3390/children8060461
Chong DY, Paley D. Deformity Reconstruction Surgery for Tibial Hemimelia. Children. 2021; 8(6):461. https://doi.org/10.3390/children8060461
Chicago/Turabian StyleChong, David Y., and Dror Paley. 2021. "Deformity Reconstruction Surgery for Tibial Hemimelia" Children 8, no. 6: 461. https://doi.org/10.3390/children8060461
APA StyleChong, D. Y., & Paley, D. (2021). Deformity Reconstruction Surgery for Tibial Hemimelia. Children, 8(6), 461. https://doi.org/10.3390/children8060461






