Identification of Impaired Executive Functioning after Pediatric Liver Transplantation Using Two Short and Easily Applicable Tests: Cognitive Functioning Module PedsQL and Children’s Color Trail Test
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Feasibility and Reliability of CCTT1 & 2 and CogPedsQL
3.2. Children’s Color Trail Test in Patients and Controls
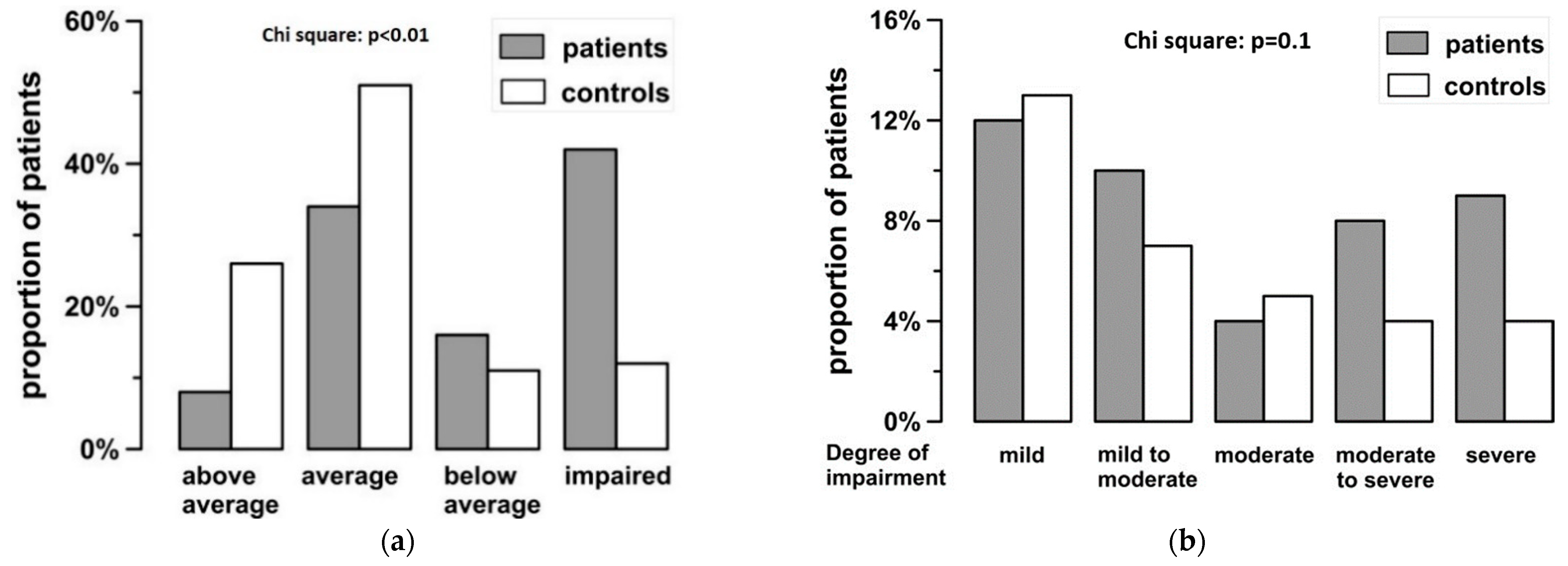
3.3. CogPeds-QL
3.4. Influence of Age and Socio-Economic Factors on Test Results
3.5. Results of Children’s Color Trail Test and CogPedsQL Are Reflected in School Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Children’s Questionnaire |
| In the past one month, how much of a problem has this been for you… Hattest Du in den vergangenen 4 Wochen folgende Probleme oder Schwierigkeiten? 1. It is hard for me to keep my attention on things. Es fiel mir schwer, mich auf eine Sache zu konzentrieren. 2. It is hard for me to remember what people tell me. Es fiel mir schwer, mich an Dinge zu erinnern, die andere mir erzählt haben. 3. It is hard for me to remember what I just heard. Es fiel mir schwer, mir Dinge zu merken, die ich gerade gehört hatte. 4. It is hard for me to think quickly. Es fiel mir schwer, schnell zu denken. 5. I have trouble remembering what I was just thinking. Ich hatte Schwierigkeiten, mich an das zu erinnern, woran ich gerade gedacht hatte. 6. I have trouble remembering more than one thing at a time. Ich hatte Schwierigkeiten, mir mehr als eine Sache gleichzeitig zu merken. |
| Parents’ Questionnaire |
| In the past one month, how much of a problem has this been for your child…. Hatte Ihr Kind in den vergangenen 4 Wochen folgende Probleme oder Schwierigkeiten? Mein Kind hatte… 1. Difficulty keeping his/her attention on things. Schwierigkeiten, sich auf eine Sache zu konzentrieren. 2. Difficulty remembering what people tell him/her Schwierigkeiten, sich an Dinge zu erinnern, die andere ihm erzählt hatten. 3. Difficulty remembering what he/she just heard Schwierigkeiten, sich Dinge zu merken, die es gerade gehört hatte. 4. Difficulty thinking quickly Schwierigkeiten, schnell zu denken. 5. Trouble remembering what he/she was just thinking Schwierigkeiten, sich an das zu erinnern, woran es gerade gedacht hatte. 6. Trouble remembering more than one thing at a time. Schwierigkeiten, sich mehr als eine Sache gleichzeitig zu merken. |
References
- Otte, J.B. Pediatric liver transplantation: Personal perspectives on historical achievements and future challenges. Liver Transpl. 2016, 22, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.M.; Uauy, R.; Waller, D.A.; Kennard, B.D.; Benser, M.; Andrews, W.S. Mental and motor development, social competence, and growth one year after successful pediatric liver transplantation. J. Pediatr. 1989, 114, 574–581. [Google Scholar] [CrossRef]
- Berquist, R.K.; Berquist, W.E.; Esquivel, C.O.; Cox, K.L.; Wayman, K.I.; Litt, I.F. Non-adherence to post-transplant care: Prevalence, risk factors and outcomes in adolescent liver transplant recipients. Pediatr. Transplant. 2008, 12, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Kennard, B.D.; Stewart, S.M.; Phelan-McAuliffe, D.; Waller, D.A.; Bannister, M.; Fioravani, V.; Andrews, W.S. Academic outcome in long-term survivors of pediatric liver transplantation. J. Dev. Behav. Pediatr. 1999, 20, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, S.; Adkins, R.; Liddell, G.A.; Jhangri, G.; Robertson, C.M. Assessment of psychoeducational outcomes after pediatric liver transplant. Am. J. Transplant. 2009, 9, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Adeback, P.; Nemeth, A.; Fischler, B. Cognitive and emotional outcome after pediatric liver transplantation. Pediatr. Transplant. 2003, 7, 385–389. [Google Scholar] [CrossRef]
- Sorensen, L.G.; Neighbors, K.; Martz, K.; Zelko, F.; Bucuvalas, J.C.; Alonso, E.M. Cognitive and academic outcomes after pediatric liver transplantation: Functional Outcomes Group (FOG) results. Am. J. Transplant. 2011, 11, 303–311. [Google Scholar] [CrossRef]
- Ohnemus, D.; Neighbors, K.; Rychlik, K.; Venick, R.S.; Bucuvalas, J.C.; Sundaram, S.S.; Ng, V.L.; Andrews, W.S.; Turmelle, Y.; Mazariegos, G.V.; et al. Health-Related Quality of Life and Cognitive Functioning in Pediatric Liver Transplant Recipients. Liver Transpl. 2020, 26, 45–56. [Google Scholar] [CrossRef]
- Krull, K.; Fuchs, C.; Yurk, H.; Boone, P.; Alonso, E. Neurocognitive outcome in pediatric liver transplant recipients. Pediatr. Transplant. 2003, 7, 111–118. [Google Scholar] [CrossRef]
- Caudle, S.E.; Katzenstein, J.M.; Karpen, S.J.; McLin, V.A. Language and motor skills are impaired in infants with biliary atresia before transplantation. J. Pediatr. 2010, 156, 936–940.e1. [Google Scholar] [CrossRef]
- Stewart, S.M.; Campbell, R.A.; McCallon, D.; Waller, D.A.; Andrews, W.S. Cognitive patterns in school-age children with end-stage liver disease. J. Dev. Behav. Pediatr. 1992, 13, 331–338. [Google Scholar] [CrossRef]
- Gilmour, S.M.; Sorensen, L.G.; Anand, R.; Yin, W.; Alonso, E.M. School outcomes in children registered in the studies for pediatric liver transplant (SPLIT) consortium. Liver Transpl. 2010, 16, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Thevenin, D.M.; Baker, A.; Kato, T.; Tzakis, A.; Fernandez, M.; Dowling, M. Neuodevelopmental outcomes for children transplanted under the age of 3 years. Transplant Proc. 2006, 38, 1692–1693. [Google Scholar] [CrossRef]
- Schulz, K.H.; Wein, C.; Boeck, A.; Rogiers, X.; Burdelski, M. Cognitive performance of children who have undergone liver transplantation. Transplantation 2003, 75, 1236–1240. [Google Scholar] [CrossRef]
- Varni, J.W.; Limbers, C.A.; Sorensen, L.G.; Neighbors, K.; Martz, K.; Bucuvalas, J.C.; Alonso, E.M. PedsQL Cognitive Functioning Scale in pediatric liver transplant recipients: Feasibility, reliability, and validity. Qual. Life Res. 2011, 20, 913–921. [Google Scholar] [CrossRef]
- Varni, J.W.; Burwinkle, T.M.; Katz, E.R.; Meeske, K.; Dickinson, P. The PedsQL in pediatric cancer: Reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer 2002, 94, 2090–2106. [Google Scholar] [CrossRef]
- Reitan, R.M.; Wolfson, D. The Trail Making Test as an initial screening procedure for neuropsychological impairment in older children. Arch. Clin. Neuropsychol. 2004, 19, 281–288. [Google Scholar] [CrossRef]
- Williams, J.; Rickert, V.; Hogan, J.; Zolten, A.J.; Satz, P.; D’Elia, L.F.; Asarnow, R.F.; Zaucha, K.; Light, R. Children’s color trails. Arch. Clin. Neuropsychol. 1995, 10, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Nichols, S.L.; Brummel, S.S.; Smith, R.A.; Garvie, P.A.; Hunter, S.J.; Malee, K.M.; Kammerer, B.L.; Wilkins, M.L.; Rutstein, R.; Tassiopoulos, K.; et al. Executive Functioning in Children and Adolescents With Perinatal HIV Infection. Pediatr. Infect. Dis. J. 2015, 34, 969–975. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Llorente, A.M.; Williams, J.; Satz, P.; D’Elia, L.F. Children’s Color Trails Test Professional Manual, 1st ed.; PAR Inc.: Lutz, FL, USA, 1998. [Google Scholar]
- Petersen, I.; Noelle, J.; Buchholz, A.; Kroencke, S.; Daseking, M.; Grabhorn, E. Fatigue in pediatric liver transplant recipients and its impact on their quality of life. Pediatr. Transplant. 2019, 23, e13331. [Google Scholar] [CrossRef] [PubMed]
- Buck, D. The PedsQL as a measure of parent-rated quality of life in healthy UK toddlers: Psychometric properties and cross-cultural comparisons. J. Child Health Care 2012, 16, 331–338. [Google Scholar] [CrossRef]
- Ee, L.C.; Lloyd, O.; Beale, K.; Fawcett, J.; Cleghorn, G.J. Academic potential and cognitive functioning of long-term survivors after childhood liver transplantation. Pediatr. Transplant. 2014, 18, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Mok, N.; Tsang, L.; Lee, T.M.; Llorente, A.M. The impact of language on the equivalence of trail making tests: Findings from three pediatric cohorts with different language dominance. Appl. Neuropsychol. 2008, 15, 123–130. [Google Scholar] [CrossRef]
- Ferrett, H.L.; Thomas, K.G.F.; Tapert, S.F.; Carey, P.D.; Conradie, S.; Cuzen, N.L.; Stein, D.J.; Fein, G. The cross-cultural utility of foreign- and locally-derived normative data for three WHO-endorsed neuropsychological tests for South African adolescents. Metab. Brain Dis. 2014, 29, 395–408. [Google Scholar] [CrossRef][Green Version]
- Ng, V.L.; Sorensen, L.G.; Alonso, E.M.; Fredericks, E.M.; Ye, W.; Moore, J.; Karpen, S.J.; Shneider, B.L.; Molleston, J.P.; Bezerra, J.A.; et al. Neurodevelopmental Outcome of Young Children with Biliary Atresia and Native Liver: Results from the ChiLDReN Study. J. Pediatr. 2018, 196, 139–147.e3. [Google Scholar] [CrossRef]
- Sorensen, L.G.; Neighbors, K.; Hardison, R.M.; Loomes, K.M.; Varni, J.W.; Ng, V.L.; Squires, R.H.; Alonso, E.M.; Bukauskas, K.; Schulte, M.; et al. Health Related Quality of Life and Neurocognitive Outcomes in the First Year after Pediatric Acute Liver Failure. J. Pediatr. 2018, 196, 129–138.e3. [Google Scholar] [CrossRef]
- Alonso, E.M.; Limbers, C.A.; Neighbors, K.; Martz, K.; Bucuvalas, J.C.; Webb, T.; Varni, J.W. Cross-sectional analysis of health-related quality of life in pediatric liver transplant recipients. J. Pediatr. 2010, 156, 270–276.e1. [Google Scholar] [CrossRef]
- Kaller, T.; Boeck, A.; Sander, K.; Richterich, A.; Burdelski, M.; Ganschow, R.; Schulz, K.H. Cognitive abilities, behaviour and quality of life in children after liver transplantation. Pediatr. Transplant. 2010, 14, 496–503. [Google Scholar] [CrossRef]
- Getsuwan, S.; Chuthapisith, J.; Treepongkaruna, S.; Butsriphum, N.; Prabpram, W.; Charoenthanakit, C.; Tanpowpong, P.; Lertudomphonwanit, C. Behavior Problems and Cognitive Function in Pediatric Liver Transplant Recipients. Transplant. Proc. 2021, 53, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, I.; van Dick, R.; Jacobi, C.; Junge, N.; Pfister, E.; Richter, N.; Baumann, U. Impact of Immunosuppression On Executive Functioning After Pediatric Liver Transplantation: An Observational Cohort Study. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 480–487. [Google Scholar] [CrossRef] [PubMed]

| n (%)/Median (Range) | |||
|---|---|---|---|
| Sex | Patients | Boys | 77 (49.7%) |
| Girls | 78 (50.3%) | ||
| Controls | Boys | 131 (44.3%) | |
| Girls | 165 (55.7%) | ||
| Age | Patients | 10.4 years (2.1–18.3) | |
| Controls | 10.0 years (2.0–18.0) n.s. | ||
| Primary disease | |||
| BA | 81 (52.3%) | ||
| Acute liver failure | 11 (7.1%) | ||
| Alpha-1-antitrypsin-deficiency | 9 (5.8%) | ||
| M. Wilson | 3 (1.9%) | ||
| AIH/PSC | 7 (4.5%) | ||
| PFIC | 7 (4.5%) | ||
| Alagille-Syndrome | 8 (5.2%) | ||
| Hepatoblastoma | 5 (3.2%) | ||
| Metabolic disease | |||
| Other (including: ARPKD (2), CF (6), neonatal hemochromatosis (2), Budd–Chiari syndrome (1), neonatal cholestasis of unknown reason (2), portal vein thrombosis (1), IFALD (1), glycogenosis (2), OTC deficiency (1), primary hyperoxaluria (1), choledochal cyst (1), and cryptogenic cirrhosis (4)) | 24 (15.5%) | ||
| Patients n (%) | Controls n (%) | p (Chi Sqare) | |
|---|---|---|---|
| Country of birth | |||
| Germany | 150 (96.8%) | 284 (95.9%) | n.s. |
| Outside Germany 1 | 1 (0.6%) | 5 (1.6%) | |
| Information missing | 4 (2.6%) | 7 (2.4%) | |
| Native Language | |||
| German | 126 (81.3%) | 261 (88.2%) | n.s. |
| Turkish | 4 (2.6 %) | 11 (3.7%) | |
| Russian | 4 (2.6%) | 3 (1.0%) | |
| Other 2 | 13 (8.3%) | 13 (4.4%) | |
| Information missing | 8 (5.2%) | 8 (2.7%) | |
| School Leaving Certificates | |||
| Mothers | |||
| None | 2 (1.3%) | 4 (1.4%) | |
| Basic level | 21 (13.5%) | 17 (5.7%) | <0.01 |
| Mid-level | 60 (38.7%) | 81 (27.4%) | |
| University entrance level | 53 (34.2%) | 180 (60.8%) | <0.01 |
| Other | 10 (6.4%) | 5 (1.7%) | |
| Information missing | 1 (0.6%) | 9 (3.0%) | |
| Fathers | |||
| None | 3 (1.9%) | 5 (1.7%) | |
| Basic level | 32 (20.6%) | 16 (5.4%) | <0.01 |
| Mid-level | 41 (26.5%) | 61 (20.6%) | |
| University entrance level | 57 (36.8%) | 180 (60.8%) | <0.01 |
| Other | 8 (5.2%) | 5 (1.7%) | |
| Information missing | 1 (0.6%) | 29 (9.8.0%) | |
| Highest Professional Degree in the Family | |||
| None | 7 (4.5%) | 10 (3.4%) | |
| Apprenticeship | 40 (25.8%) | 47 (15.9%) | <0.01 |
| Vocational school | 39 (25.1%) | 46 (15.6%) | <0.01 |
| University of cooperative | 17 (11.0%) | 41 (13.9%) | |
| Education | |||
| University degree | 24 (15.5%) | 81 (27.4%) | <0.01 |
| PhD | 10 (6.5%) | 55 (18.6%) | <0.01 |
| Other | 6 (3.9%) | 1 (0.3%) | |
| Information missing | 12 (7.7%) | 15 (5.1%) |
| Patients | Controls | p | Cohen’s d | |
|---|---|---|---|---|
| CCTT1 | ||||
| All | 81.5 ± 19.3 n = 84 | 95.3 ± 15.1 n = 191 | <0.01 | −0.84 |
| age 8–12 | 77.1 ± 17.8 n = 40 | 94.2 ± 15.1 n = 115 | <0.01 | −1.1 |
| age 13–16 | 85.5 ± 19.9 n = 44 | 96.9 ± 15.2 n = 76 | <0.01 | −0.67 |
| 8–12 vs. 13–16 p = 0.04 d = −0.44 | 8–12 vs. 13–16 p = 0.22 n.s. | |||
| CCTT2 | ||||
| All | 87.8 ± 15.6 n = 85 | 98.7 ± 11.9 n = 191 | <0.01 | −0.83 |
| age 8–12 | 86.9 ± 16.3 n = 40 | 99.3 ± 11.3 n = 115 | <0.01 | −0.97 |
| age 13–16 | 88.7 ± 15.1 n = 45 | 97.1 ± 12.7 n = 76 | <0.01 | −0.62 |
| 8–12 vs. 13–16 p = 0.6 n.s. | 8–12 vs. 13–16 p = 0.14 n.s. |
| Patients cog-PedsQL Children | Controls cog-PedsQL Children | p Patients vs. Controls | Cohen’s d | |
| PedsQL Children | ||||
| All | 66.8 ± 20.5 (n = 121) | 73.9 ± 17.1 (n = 247) | <0.01 | −0.39 |
| age 5–7 | 50.5 ± 22.0 (n = 24) | 61.6 ± 22.6 (n = 54) | 0.046 | −0.49 |
| age 8–12 | 68.9 ± 19.5 (n = 42) | 78.1 ± 13.5 (n = 115) | <0.01 | −0.60 |
| age 13–18 | 72.4 ± 16.8 (n = 55) | 76.4 ± 13.3 (n = 78) | 0.13 n.s. | −0.27 |
| 5–7 vs. 8–12 | p < 0.01, d = −0.90 | p < 0.01, d = −0.98 | ||
| 5–7 vs. 13–18 | p < 0.01, d = −1.18 | p < 0.01, d = −0.84 | ||
| 8–12 vs. 13–18 | 0.34 n.s., d = −0.19 | 0.39 n.s., d = 0.13 | ||
| Patients Parent proxy cogPesQL | Controls Parent proxy cog-PedsQL | p Patients vs. Controls | ||
| PedsQL Parents | ||||
| All | 65.2 ± 23.5 (n = 147) | 79.2 ± 16.6 (n = 279) | <0.01 | −0.73 |
| Age 2–4 | 74.7 ± 22.5 (n = 25) | 75.5 ± 14.2 (n = 29) * | 0.85 n.s. | −0.04 |
| age 5–7 | 61.9 ± 21.1 (n = 29) | 80.2 ± 15.7 (n = 55) * | <0.01 | −1.03 |
| age 8–12 | 55.7 ± 22.8 (n = 39) | 80.7 ± 16.1 (n = 110) * | <0.01 | −1.38 |
| age 13–18 | 69.3 ± 23.3 (n = 54) | 79.17 ± 18.2 (n = 74) * | 0.01 | −0.48 |
| 2–4 vs. 5–7 | p = 0.035, d = 0.59 | p = 0.18 n.s. | ||
| 2–4 vs. 8–12 | p = 0.002, d = 0.84 | p = 0.11 n.s. | ||
| 2–4 vs. 13–18 | p = 0.34 n.s. | p = 0.33 n.s. | ||
| 5–7 vs. 8–12 | p = 0.26 n.s. | p = 0.83 n.s. | ||
| 5–7 vs. 13–18 | p = 0.15 n.s. | p = 0.74 n.s. | ||
| 8–12 vs. 13–18 | p = 0.006, d = −0.59 | p = 0.55 n.s. | ||
| Patients Paired cog-PedsQL Children vs. Parents | Controls Paired cog-PedsQL Children vs. Parents | |||
| All | 67.3 ± 20.3 vs. 63.8 ± 22.7 (n = 116) p = 0.11, n.s. | 74.3 ± 16.9 vs. 79.9 ± 16.7 (n = 231) p < 0.01, d = −0.33 | ||
| age 5–7 | 51.2 ± 22.2 vs. 65.9 ± 16.1 (n = 23) p = 0.012, d = 0.76 | 61.7 ± 22.4 vs. 79.2 ± 16.0 (n = 50) p < 0.01, d = −0.89 | ||
| age 8–12 | 69.2 ± 19.9 vs. 55.9 ± 23.1 (n = 38) p < 0.01, d = 0.62 | 78.6 ± 13.0 vs. 80.5 ± 16.1 (n = 109) p = 0.26 n.s. | ||
| age 13–18 | 72.9 ± 16.4 vs. 69.3 ± 23.5 (n = 53) p = 0.16, n.s. | 76.4 ± 13.4 vs. 79.3 ± 18.1 (n = 72) p = 0.19, n.s. |
| Mother | Father | |||
|---|---|---|---|---|
| Level of Secondary Education | Level of Tertiary Education | Level of Secondary Education | Level of Tertiary Education | |
| CCTT1 | ||||
| Patients | 0.1 | 0.05 | 0.19 | 0.25 |
| Controls | 0.02 | 0.00 | 0.00 | −0.05 |
| CCTT2 | ||||
| Patients | 0.09 | −0.02 | 0.06 | 0.05 |
| Controls | 0.04 | 0.11 | 0.12 | 0.17 |
| cogPedsQL Children | ||||
| Patients | 0.09 | 0.27 ** | 0.11 | 0.12 |
| Controls | 0.16 ** | 0.14 * | 0.14 * | 0.16 * |
| cogPedsQL Parents | ||||
| Patients | 0.05 | 0.18 * | 0.05 | 0.07 |
| Controls | 0.26 ** | 0.19 ** | 0.24 ** | 0.22 ** |
| Mother | Father | |||
|---|---|---|---|---|
| Level of Secondary Education | Level of Tertiary Education | Level of Secondary Education | Level of Tertiary Education | |
| School performance | ||||
| Patients | −0.21 * | −0.03 | −0.11 | −0.05 |
| Controls | −0.26 ** | −0.26 ** | −0.26 ** | −0.21 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldschmidt, I.; van Dick, R.; Jacobi, C.; Pfister, E.D.; Baumann, U. Identification of Impaired Executive Functioning after Pediatric Liver Transplantation Using Two Short and Easily Applicable Tests: Cognitive Functioning Module PedsQL and Children’s Color Trail Test. Children 2021, 8, 571. https://doi.org/10.3390/children8070571
Goldschmidt I, van Dick R, Jacobi C, Pfister ED, Baumann U. Identification of Impaired Executive Functioning after Pediatric Liver Transplantation Using Two Short and Easily Applicable Tests: Cognitive Functioning Module PedsQL and Children’s Color Trail Test. Children. 2021; 8(7):571. https://doi.org/10.3390/children8070571
Chicago/Turabian StyleGoldschmidt, Imeke, Rolf van Dick, Christoph Jacobi, Eva Doreen Pfister, and Ulrich Baumann. 2021. "Identification of Impaired Executive Functioning after Pediatric Liver Transplantation Using Two Short and Easily Applicable Tests: Cognitive Functioning Module PedsQL and Children’s Color Trail Test" Children 8, no. 7: 571. https://doi.org/10.3390/children8070571
APA StyleGoldschmidt, I., van Dick, R., Jacobi, C., Pfister, E. D., & Baumann, U. (2021). Identification of Impaired Executive Functioning after Pediatric Liver Transplantation Using Two Short and Easily Applicable Tests: Cognitive Functioning Module PedsQL and Children’s Color Trail Test. Children, 8(7), 571. https://doi.org/10.3390/children8070571






