Gestation-Based Viability–Difficult Decisions with Far-Reaching Consequences
Abstract
:1. Historical Perspective
2. Gestational Maturity
3. Counseling
4. Postnatal Care
5. Survival to Discharge
6. Ethics
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Liu, L.; Johnson, H.L.; Cousens, S.; Perin, J.; Scott, S.; Lawn, J.; Rudan, I.; Campbell, H.; Cibulskis, R.; Li, M.; et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 2012, 379, 2151–2161. [Google Scholar] [CrossRef]
- Smith, L.K.; Morisaki, N.; Morken, N.-H.; Gissler, M.; Deb-Rinker, P.; Rouleau, J.; Håkansson, S.; Kramer, M.; Kramer, M.S. An International Comparison of Death Classification at 22 to 25 Weeks’ Gestational Age. Pediatr. 2018, 142, e20173324. [Google Scholar] [CrossRef] [Green Version]
- Philip, A.G.S. The Evolution of Neonatology. Pediatr. Res. 2005, 58, 799–815. [Google Scholar] [CrossRef]
- Younge, N.; Goldstein, R.F.; Bann, C.; Hintz, S.R.; Patel, R.; Smith, P.B.; Bell, E.F.; Rysavy, M.; Duncan, A.F.; Vohr, B.R.; et al. Survival and Neurodevelopmental Outcomes among Periviable Infants. N. Engl. J. Med. 2017, 376, 617–628. [Google Scholar] [CrossRef] [Green Version]
- Serenius, F.; Källén, K.; Blennow, M.; Ewald, U.; Fellman, V.; Holmström, G.; Lindberg, E.; Lundqvist, P.; Maršál, K.; Norman, M.; et al. Neurodevelopmental Outcome in Extremely Preterm Infants at 2.5 Years After Active Perinatal Care in Sweden. JAMA 2013, 309, 1810–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kono, Y.; Yonemoto, N.; Nakanishi, H.; Kusuda, S.; Fujimura, M. Changes in survival and neurodevelopmental outcomes of infants born at <25 weeks’ gestation: A retrospective observational study in tertiary centres in Japan. BMJ Paediatr. Open 2018, 2, e000211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Söderström, F.; Normann, E.; Jonsson, M.; Ågren, J. Outcomes of a uniformly active approach to infants born at 22–24 weeks of gestation. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Watkins, P.L.; Dagle, J.M.; Bell, E.F.; Colaizy, T.T. Outcomes at 18 to 22 months of corrected age for infants born at 22 to 25 weeks of gestation in a center practicing active management. J. Pediatr. 2020, 217, 52–58. [Google Scholar] [CrossRef]
- Di Stefano, L.M.; Wood, K.; Mactier, H.; Bates, S.E.; Wilkinson, D. Viability and thresholds for treatment of extremely preterm infants: Survey of UK neonatal professionals. Arch. Dis. Child. Fetal Neonatal Ed. 2021. [Google Scholar] [CrossRef]
- Janvier, A.; Baardsnes, J.; Hebert, M.; Newell, S.; Marlow, N. Variation of practice and poor outcomes for extremely low gestation births: Ordained before birth? Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F470–F471. [Google Scholar] [CrossRef] [Green Version]
- Welty, S. Challenging the gestational age for the limit of viability: Proactive care. J. Perinatol. 2018, 39, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.H.; Söderström, F.; Ågren, J.; Sindelar, R.; Bartlett, C.; Rivera, B.K.; Mitchell, C.C.; Frey, H.A.; Shepherd, E.G.; Nelin, L.D.; et al. Outcomes following a comprehensive versus a selective approach for infants born at 22 weeks of gestation. J. Perinatol. 2018, 39, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Mehler, K.; Oberthuer, A.; Keller, T.; Becker, I.; Valter, M.; Roth, B.; Kribs, A. Survival Among Infants Born at 22 or 23 Weeks’ Gestation Following Active Prenatal and Postnatal Care. JAMA Pediatr. 2016, 170, 671–677. [Google Scholar] [CrossRef] [Green Version]
- Zayek, M.M.; Trimm, R.F.; Hamm, C.R.; Peevy, K.J.; Benjamin, J.T.; Eyal, F.G. The Limit of Viability. Arch. Pediatr. Adolesc. Med. 2011, 165, 126–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, N.; Kono, Y.; Yonemoto, N.; Kusuda, S.; Fujimura, M. Outcomes of Infants Born at 22 and 23 Weeks’ Gestation. Pediatrics 2013, 132, 62–71. [Google Scholar] [CrossRef] [Green Version]
- Norman, M.; Hallberg, B.; Abrahamsson, T.; Björklund, L.J.; Domellöf, M.; Farooqi, A.; Bruun, C.F.; Gadsbøll, C.; Hellström-Westas, L.; Ingemansson, F.; et al. Association Between Year of Birth and 1-Year Survival Among Extremely Preterm Infants in Sweden During 2004–2007 and 2014–2016. JAMA 2019, 321, 1188–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janvier, A.; Barrington, K.J.; Payot, A. A time for hope: Guidelines for the perinatal management of extremely preterm birth. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 230–231. [Google Scholar] [CrossRef] [PubMed]
- Costeloe, K.L.; Hennessy, E.M.; Haider, S.; Stacey, F.; Marlow, N.; Draper, E.S. Short term outcomes after extreme preterm birth in England: Comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ 2012, 345, e7976. [Google Scholar] [CrossRef] [Green Version]
- Raju, T.N.; Mercer, B.M.; Burchfield, D.J.; Joseph, G.F., Jr. Periviable birth: Executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. Obs. Gynecol. 2014, 123, 1083–1096. [Google Scholar]
- Lemyre, B.; Moore, G. Canadian Paediatric Society, Fetus and Newborn Committee. Counselling and management for antici-pated extremely preterm birth. Paediatr. Child Health 2017, 22, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Nuffield Council on Bioethics: Critical Care Decisions in Fetal and Neonatal Medicine: Ethical Issues. November 2006. Available online: https://www.nuffieldbioethics.org/assets/pdfs/Critical-care-decisions.pdf (accessed on 15 June 2021).
- ACOG Practice Bulletin No. 98: Ultrasonography in Pregnancy. Obs. Gynecol. 2008, 112, 951.
- Canadian Neonatal Network. Annual Reports. 2016. Available online: http://www.canadianneonatalnetwork.org/Portal (accessed on 31 July 2018).
- Costeloe, K.; Hennessy, E.; Gibson, A.T.; Marlow, N.; Wilkinson, A.R.; for the EPICure Study Group. The EPICure Study: Outcomes to Discharge From Hospital for Infants Born at the Threshold of Viability. Pediatrics 2000, 106, 659–671. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.; Van Dyk, J.; Zein, H.; Aguirre, A.N.; Hendson, L.; Church, P.; Banihani, R.; Asztalos, E. Split-week gestational age model provides valuable information on outcomes in extremely preterm infants. Acta Paediatr. 2020, 109, 2578–2585. [Google Scholar] [CrossRef]
- McElrath, T.F.; Robinson, J.N.; Ecker, J.L.; Ringer, S.A.; Norwitz, E.R. Neonatal outcome of infants born at 23 weeks’ gestation. Obstet. Gynecol. 2001, 97, 49–52. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Amon, E.; Al-Hosni, M.; Gavard, J.A.; Gross, G.; Myles, T.D. ‘Early’ versus ‘late’ 23-week infant outcomes. Am. J. Obstet. Gynecol. 2012, 207, 226-e1. [Google Scholar] [CrossRef] [PubMed]
- Staub, K.; Baardsnes, J.; Hébert, N.; Hebert, M.; Newell, S.; Pearce, R. Our child is not just a gestational age. A first-hand account of what parents want and need to know before premature birth. Acta Paediatr. 2014, 103, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Zdanowicz, J.A.; Huber, C.; Gerull, R.; Mueller, M.; Raio, L.; Surbek, D. Impact of Fetal Weight Estimation on the Prediction of Neonatal Morbidity and Mortality at the Limit of Viability. Fetal Diagn. Ther. 2016, 42, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, H.; Ochiai, M.; Yasuoka, K.; Tanaka, K.; Kurata, H.; Fujiyoshi, J.; Matsushita, Y.; Suga, S.; Nonaka, K.; Taguchi, T.; et al. Early Mortality and Morbidity in Infants with Birth Weight of 500 Grams or Less in Japan. J. Pediatr. 2017, 190, 112–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, D. Who should decide for critically ill neonates and how? The grey zone in neonatal treatment decisions. In When Doctors and Parents Disagree: Ethics, Paediatrics and the Zone of Parental Discretion; McDougall, R., Delany, C., Gillam, L., Eds.; The Federation Press: Sydney, Australia, 2016. [Google Scholar]
- Handley, S.C.; Sun, Y.; Wyckoff, M.H.; Lee, H.C. Outcomes of extremely preterm infants after delivery room cardiopulmonary resuscitation in a population-based cohort. J. Perinatol. 2015, 35, 379–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorner, R.A.; Burton, V.J.; Allen, M.C.; Robinson, S.; Soares, B.P. Preterm neuroimaging and neurodevelopmental outcome: A focus on intraventricular hemorrhage, post-hemorrhagic hydrocephalus, and associated brain injury. J. Perinatol. 2018, 38, 1431–1443. [Google Scholar] [CrossRef]
- Janvier, A.; Leuthner, S.R. Chronic patients, burdensome interventions and the Vietnam analogy. Acta Paediatr. 2013, 102, 669–670. [Google Scholar]
- Moro, T.T.; Kavanaugh, K.; Savage, T.A.; Reyes, M.R.; Kimura, R.E.; Bhat, R. Parent Decision Making for Life Support for Extremely Premature Infants. J. Périnat. Neonatal Nurs. 2011, 25, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Mactier, H.; Bates, S.E.; Johnston, T.; Lee-Davey, C.; Marlow, N.; Mulley, K.; Smith, L.; To, M.; Wilkinson, D. Perinatal management of extreme preterm birth before 27 weeks of gestation: A framework for practice. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Amer, R.; Moddemann, D.; Seshia, M. Canadian Neonatal Network and Canadian Neonatal Follow-up Network Investigators: Neurodevelopmental outcomes of infants born at <29 weeks of gestation admitted to Canadian Neonatal Intensive Care Units based on location of birth. J. Pediatr 2018, 196, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Shipley, L.; Gyorkos, T.; Dorling, J.; Tata, L.; Szatkowski, L.; Sharkey, D. Risk of Severe Intraventricular Hemorrhage in the First Week of Life in Preterm Infants Transported Before 72 Hours of Age*. Pediatr. Crit. Care Med. 2019, 20, 638–644. [Google Scholar] [CrossRef]
- Goswami, I.; Redpath, S.; Langlois, R.; Green, J.; Lee, K.; Whyte, H. Whole-body vibration in neonatal transport: A review of current knowledge and future research challenges. Early Hum. Dev. 2020, 146, 105051. [Google Scholar] [CrossRef]
- Redpath, S. Canadian Neonatal Transport Network and Canadian Neonatal Network Investigators; Shah, P.S.; Moore, G.P.; Yang, J.; Toye, J.; Perreault, T.; Lee, K.-S. Do transport factors increase the risk of severe brain injury in outborn infants <33 weeks gestational age? J. Perinatol. 2020, 40, 385–393. [Google Scholar] [CrossRef]
- Agostoni, C.; Buonocore, G.; Carnielli, V.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellöf, M.; Embleton, N.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral Nutrient Supply for Preterm Infants: Commentary From the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, D.C.; García, S.S.; Renau, M.I.; Iglesias-Platas, I. Availability of Donor Milk for Very Preterm Infants Decreased the Risk of Necrotizing Enterocolitis without Adversely Impacting Growth or Rates of Breastfeeding. Nutrients 2019, 11, 1895. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, B.; Asztalos, E.V.; Roberts, R.S.; Robertson, C.M.T.; Sauve, R.S.; Whitfield, M.F.; for the Trial of Indomethacin Prophylaxis in Preterms (TIPP) Investigators. Impact of Bronchopulmonary Dysplasia, Brain Injury, and Severe Retinopathy on the Outcome of Extremely Low-Birth-Weight Infants at 18 Months: Results from the Trial of Indomethacin Prophylaxis in Preterms. JAMA 2003, 289, 1124–1129. [Google Scholar] [CrossRef] [Green Version]
- Isayama, T. The clinical management and outcomes of extremely preterm infants in Japan: Past, present, and future. Transl. Pediatr. 2019, 8, 199–211. [Google Scholar] [CrossRef]
- Shah, P.S.; Lui, K.; Sjörs, G.; Mirea, L.; Reichman, B.; Adams, M.; Modi, N.; Darlow, B.A.; Kusuda, S.; Feliciano, L.S.; et al. Neonatal Outcomes of Very Low Birth Weight and Very Preterm Neonates: An International Comparison. J. Pediatr. 2016, 177, 144–152. [Google Scholar] [CrossRef]
- Miller, J.E.; Hammond, G.C.; Strunk, T.; Moore, H.C.; Leonard, H.; Carter, K.W.; Bhutta, Z.; Stanley, F.; De Klerk, N.; Burgner, D. Association of gestational age and growth measures at birth with infection-related admissions to hospital throughout childhood: A population-based, data-linkage study from Western Australia. Lancet Infect. Dis. 2016, 16, 952–961. [Google Scholar] [CrossRef] [Green Version]
- Shankaran, S.; Fanaroff, A.A.; Wright, L.L.; Stevenson, D.K.; Donovan, E.F.; Ehrenkranz, R.A.; Langer, J.C.; Korones, S.B.; Stoll, B.J.; Tyson, J.E.; et al. Risk factors for early death among extremely low-birth-weight infants. Am. J. Obstet. Gynecol. 2002, 186, 796–802. [Google Scholar] [CrossRef]
- Nayak, B.; Moon, J.-Y.; Kim, M.; Fischhoff, B.; Haward, M.F. Optimism bias in understanding neonatal prognoses. J. Perinatol. 2021, 41, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Partridge, E.A.; Davey, M.G.; Hornick, M.A.; McGovern, P.E.; Mejaddam, A.Y.; Vrecenak, J.D.; Mesas-Burgos, C.; Olive, A.; Caskey, R.C.; Weiland, T.R.; et al. An extra-uterine system to physiologically support the extreme premature lamb. Nat. Commun. 2017, 8, 15112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
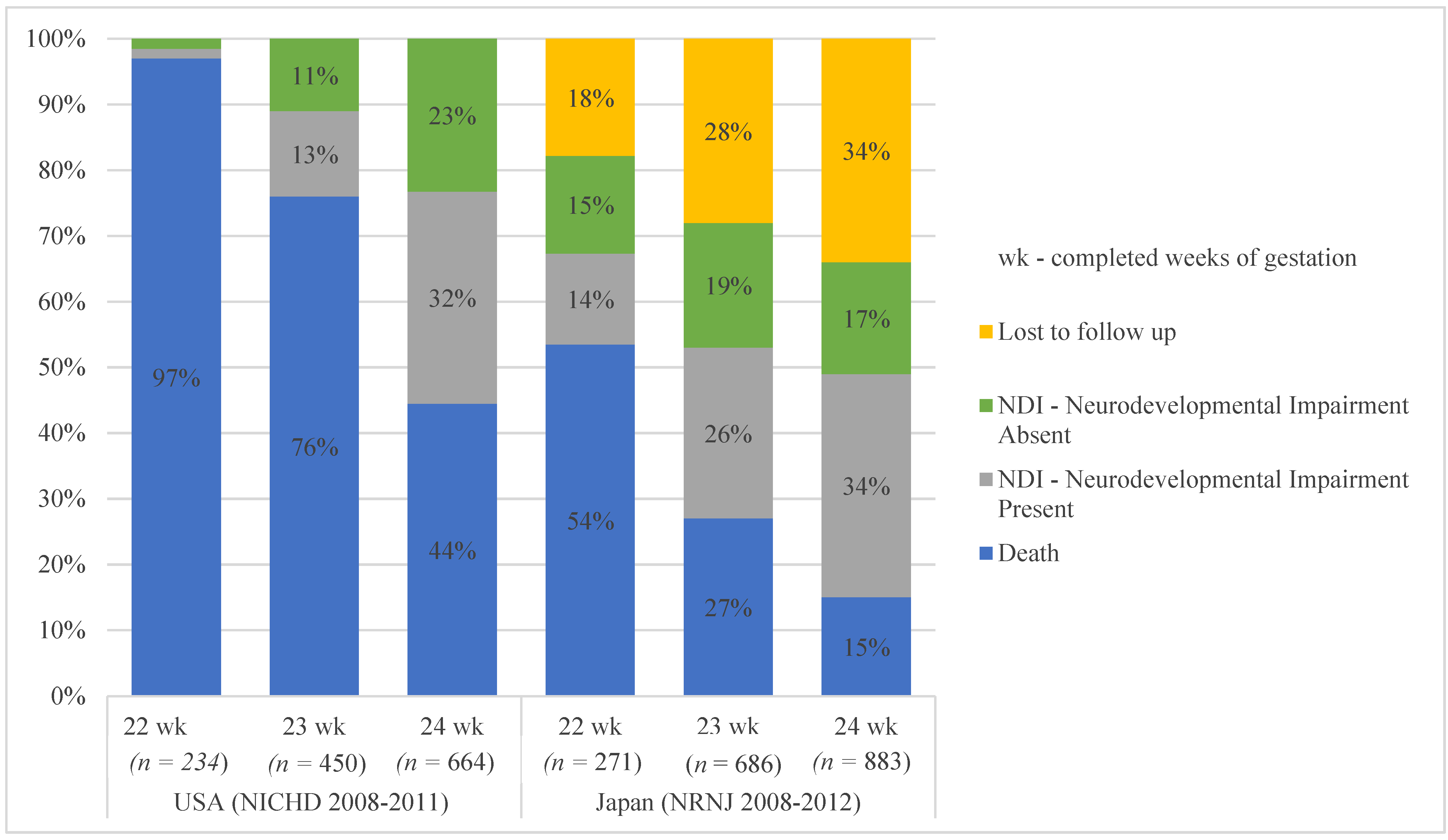

| Viability | Active Resuscitation of Infants at 22–23 Weeks Gestational Age |
|---|---|
| Circulatory Care | Guided by neonatologist-performed echocardiography |
| Respiratory care | Reliance on early invasive ventilation |
| Neuroprotection | (I) Minimal handling; (II) sedation for ventilated infants; (III) serial cranial ultrasounds |
| Nutritional care | (I) Promotion of breastfeeding; (II) early minimal enteral feeding; (III) routine use of glycerin enema; (IV) use of probiotics |
| Infection | (I) Gloves, masks, gowns for patient care; (II) serial CRP monitoring |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, S.; Asztalos, E. Gestation-Based Viability–Difficult Decisions with Far-Reaching Consequences. Children 2021, 8, 593. https://doi.org/10.3390/children8070593
Thomas S, Asztalos E. Gestation-Based Viability–Difficult Decisions with Far-Reaching Consequences. Children. 2021; 8(7):593. https://doi.org/10.3390/children8070593
Chicago/Turabian StyleThomas, Sumesh, and Elizabeth Asztalos. 2021. "Gestation-Based Viability–Difficult Decisions with Far-Reaching Consequences" Children 8, no. 7: 593. https://doi.org/10.3390/children8070593
APA StyleThomas, S., & Asztalos, E. (2021). Gestation-Based Viability–Difficult Decisions with Far-Reaching Consequences. Children, 8(7), 593. https://doi.org/10.3390/children8070593






