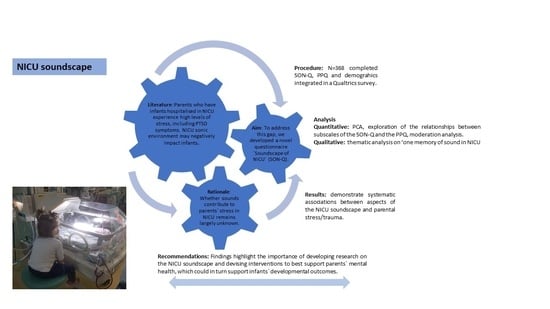
The Soundscape of Neonatal Intensive Care: A Mixed-Methods Study of the Parents’ Experience
Abstract
1. Introduction
2. Method
2.1. Participants
2.2. Materials
- (i)
- The sound in the NICU questionnaire—Soundscape of NICU Questionnaire (henceforth SON-Q)—was developed through the consultation with parents and relevant professionals. The questionnaire was designed to cover the following areas: (1) About your baby (demographics about infants, collected at the start of the questionnaire); (2) You and sound: Going to an NICU; (3) Your baby and sound in the NICU; (4) At home after being in the NICU; (5) About the NICU in general; and about you (demographics about parents, collected at the end of the questionnaire). Overall, the SON-Q included 32 questions articulated in 204 items evaluated on a 5-point Likert scale. Given that the SON-Q was long, due to its exploratory nature, we deemed it important to include some negatively worded items (e.g., “I did not notice vocalisations”) to introduce some “checks” of respondents’ sincerity and avoid acquiescence bias, thus increasing reliability [89]. Section (4) also presented one open question (optional), in which parents could share a memory they had from their experience in NICUs, which was associated with sound.
- (ii)
- The PPQ was used to investigate the presence of PTSD symptoms in the parents. The PPQ comprises of 14 items scored on a five-point scale, ranging from 0 = not at all to 4 = often, more than a month (e.g., Did you have bad dreams of giving birth or of your baby’s hospital stay?). Higher scores are indicative of more severe PTSD symptomatology. There are also three subscales measured: intrusion symptoms, avoidance symptoms and hyperarousal symptoms.
2.3. Procedure
2.4. Data Analysis
2.5. Ethics
3. Results
3.1. SON-Q Quantitative Analyses Results
3.2. Open Question Qualitative Analysis
- Theme 1: Sounds of machines and various inanimate objects: What’s a “beep”?
“The soft beeping of the monitors is overwhelmingly etched in my memory” (P92).
“I’ll never forget the high pitch patterned beep if the UV light for jaundice and the alarm when heart rate went to low or oxygen went low” (P213).
“The sound of the doors opening; the sound of shoes on the highly buffed floors” (P100).
- Constant “beeping” as a symbol of the parents’ ceaseless worrying.
“The relentlessness of the sounds was overwhelming, but I think more than the sounds alone, was what they represented-danger; sickness; probable death. And it was that combination that I found torturous” (P176).
“During my son’s NICU stay his condition deteriorated rapidly. His belly was swollen and his oxygen saturation’s suddenly dropped whilst on bipap breathing support. The machines were getting louder and louder, doctors were running around taking turns to try and stabilise him. Another doctor was on the phone to a higher level NICU hospital for an emergency transfer. No one could tell me what was going on. Between the noisy machines, not being able to be near my son as there were four doctors working on him, the noise from doctors & nurses rushing. I was completely overwhelmed” (P34).
“I remember that every time the emergency alarm was on I felt that my world is breaking. I would let anything behind, run to my babies’ nursery and pray that they were well, and the alarm was not on because they needed help” (P1).
“Beeping. Incessant beeping. Of different tones and pitches but beeping. One time my baby’s oxygen monitor went off whilst I was feeding her but I couldn’t differentiate this as urgent, compared to all the other beeps that we going on” (P78).
“Alarms going off is associated with my babies desats. I think I have PTSD according to my reaction to alarms” (P58).
“The Asystole and bradycardia and Apnea alarms haunt me” (P74).
“The low loud “boom boom” sound that the monitors made will stay with me forever. Whenever I look back at recordings of my son and I hear that noise in the background it makes me well up. It’s a haunting sound” (P117).
“Once a very sick baby crashed 3 times in 25 min in our ICU room. It was terrifying. I still have nightmares almost 2 yrs later. The crash alarm rings in my ears so loud. I’ll never forget it as long as I live” (P232).
“Regular beeping noise still create feelings of stress and anxiety for me” (P210).
“I just remember that for many years my son was terrified of sudden high pitches noises. He could go from floor straight into my arms at one leap if he heard something like a monitor sound. But could sleep through a Hoover next to his cot!” (P174).
“Sounds are scary to start with but become reassuring” (P192).
“For a while after coming home, I’d have to put the radio on quietly in the bedroom. Bings and bongs were somewhat reassuring” (P149).
“My son used to suffer from desats where the monitor used to alarm if it happened which was quite frequent. When it was being discussed he could go home they turned off this monitor just a few days before discharge which I felt gave me anxiety because I got into a habit of relying on the noise to tell me when it was happening” (P124).
“When returning home in the evenings, I would constantly hear all the beeping from the machines in the NICU, on occasion I would wake up after hearing the urgent call response (although I was at home) prompting me to ring the NICU throughout the night. The noises from the machines were so reassuring yet so traumatic” (P138).
“The sounds of the monitors became familiar & strangely comforting, especially when I visited our daughter alone” (P118).
- Theme 2: Sounds from human sources.
- The sounds of medical staff.
“My daughter was very sensitive to sound. Even with signs reminding staff to talk softly they would talk very loudly, and her stats would drop” (P62).
“Staff talking too loud/laughing” (P11).
“Staff talking loudly and often about non-work-related matters” (P115).
- The sounds of other hospitalised families.
“Visitors of one of the babies were regularly very loud with children running and screaming which visibly affected my baby” (P132).
“Staff (rightly) weren’t in control of the volume of other parents, some at times were loudly derogatory about staff and I felt so torn about leaving my baby and felt so guilty knowing I was leaving her in such a toxic environment (thankfully this was the minority (but loudest!) of parents” (P 99).
“Other parents were loud” (P146).
- Theme 3: Unheard parents’ sounds: “we couldn’t vocalise!”.
- Parents’ feelings of transparency and incapability.
”I found it difficult to connect with my baby as it was too quiet, and I didn’t want to disturb the rest of the unit at times.” (P82).
“So quiet around us that we preferred not to talk rather than have everyone hear us. I was embarrassed and overwhelmed and just went into myself. I didn’t want to be there and I didn’t know what I was doing” (P88).
“I hadn’t held my baby and didn’t let myself feel anything for him so there was no questions of me singing or chatting to him in that situation. I had to wait until I was at home alone with him for that” (P88).
“… the fear of speaking too loud and/or making noise. Feeling like you had to be silent” (P166).
“My baby was taken off me by the NICU nurse and rubbed and manitoulated (sic) until the oxygen levels were restored. I’ve thought about that moment a lot that I could and should have done more but I didn’t because I didn’t know what this beeping was telling me” (P78).
- Parents’ perplexity towards other babies’ cry.
“The most heart-breaking noise for me was the crying of other babies when nobody was around to attend to them. I worried that when my daughter started to cry, she would be left in the same way. I really didn’t mind the alarms, but I hated hearing the other babies cry” (P204).
“…staff would often not have time meaning babies next to mine sat crying or their monitors went off and nobody appeared to look at them, it made me anxious did they ignore my baby when I wasn’t there?“ (P194).
“The crying of other people’s babies made me feel frustrated that we could not be at home enjoying each other’s company in our own bubble. As a first-time parent, you expect to enjoy a certain time of calm and to be used to your new arrival, the NICU attacks all your senses and everything you have prepared your baby for. The sounds are as unfamiliar to baby as they are to you” (P188).
- Theme 4: The sound of music as balancing the NICU’s cacophony.
- Music as a supporting agent for parents and babies in the NICU.
“I remember my baby’s night nurse would sing and hum as she did her rounds. It seems like when she hummed during feedings, the babies would take to the bottles better. When she hummed my baby would turn in her direction, or her heart rate would even out while she was being held and hummed to by her (much like she did being held by her parents). Sound is very important to babies, especially those in loud NICUs, in order to maintain a peaceful demeanor” (P68).
“Special care unit towards the end of our stay started to play music in the corridors between the nursery and it made all the difference to the parents as it made it feel less like a hospital setting” (P38).
“Whilst pregnant, I played piano everyday (I play to a high standard) and got heavily into a particular composer. His work will be forever associated with that time as after my daughter’s birth, I downloaded an album of his played work and played it to her throughout NICU and beyond. Her experience of NICU was very calm-she slept through it. Literally. I believe it was aided by as much kangaroo care as I could give, and music. Non-stop music that the nurses let me play to her” (P43).
“It seems like when she hummed during feedings, the babies would take to the bottles better” (P68).
- Music as a transitional object for families when entering their home environments.
“My husband and I slept with music on for two months after bringing our daughter home” (P197).
“There is a song by a band Athlete called ‘Wires’ written about their experience in NICU. Every time I hear it, I bawl my eyes out. Certain noises or in this instance songs bring back the vivid memories” (P154).
“I loved being able to sit in a rocking chair with my son with my worship music playing. He was the most active while I was pregnant listening to that music and the most relaxed in the NICU and once home” (P61).
4. Discussion
5. Conclusions
- (i)
- It is important to address parents’ needs in order to support their mental health. For instance, being delivered in a family-centred approach in combination with Kangaroo Care, music therapy in NICUs has been shown to be beneficial, helping to reduce parents’ anxiety and stress and improve their mood, restfulness and motivation [136]. Additionally, it improves breastfeeding [137], relaxation [138] and parent–infant bonding [100,139], which is supported by emerging parental identity [140].
- (ii)
- Future research is important to extend this study cross-culturally and to investigate the sonic experience of premature infants and their parents during both their NICU stay and early postdischarge across a variety of cultural and social contexts [141,142,143,144]. The convergent quantitative/qualitative results demonstrated the importance of opening a conversation between parents who have experience in NICUs, medical and nursing staff, psychologists and engineers, to plan strategies for improving the sonic experience in NICUs. This is crucial for both infants and parents, encompassing support for parent–infant vocal interaction and the mitigation of noxious aspects of the NICU soundscape, especially those derived from medical machines.
- (iii)
- In this respect, future research may involve partnerships with technology developers targeting the overall improvement of the NICU soundscape. The present study focuses on the subjective parental experience with the NICU soundscape, but two important points need to be kept in mind. Indeed, different brands of the same equipment may use more/less loud or unpleasantly pitched signals and hence may affect the NICU soundscape in different ways; similarly, the layout and space available in the wards may also increase/diffuse noxious sound effects. Last but not least, the noisy equipment in NICUs in many cases has a life-saving function, and hence, the relevant question is not about having or not having the equipment, but regarding implementing changes in which signals could be designed either based on current understandings of human emotional responses to sounds with different characteristics or based on the exploration of other sensorial modalities (e.g., vibration) or a combination of sensorial modalities allowing for different levels of sensitivity. Objective measures of specific acoustical parameters should be the basis for new standards and future interdisciplinary studies comparing outcomes in both infants (e.g., auditory development) and parents (e.g., perinatal stress affecting their parenting ability and coping) across environments.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vouloumanos, A.; Hauser, M.D.; Werker, J.F.; Martin, A. The tuning of human neonates’ preference for speech. Chil. Dev. 2010, 81, 517–527. [Google Scholar] [CrossRef]
- DeCasper, A.J.; Spence, M.J. Prenatal maternal speech influences newborns’ perception of speech sounds. Inf. Behav. Dev. 1986, 9, 133–150. [Google Scholar] [CrossRef]
- DeCasper, A.J.; Lecanuet, J.P.; Busnel, M.C.; Granier-Deferre, C.; Maugeais, R. Fetal reactions to recurrent maternal speech. Inf. Behav. Dev. 1994, 17, 159–164. [Google Scholar] [CrossRef]
- Kisilevsky, B.S.; Hains, S.M.J.; Jacquet, A.Y.; Granier-Deferre, C.; Lecanuet, J.P. Maturation of fetal responses to music. Dev. Sci. 2004, 7, 550–559. [Google Scholar] [CrossRef]
- Lecanuet, J.P.; Granier-Deferre, C.; Jacquet, A.Y.; Capponi, I.; Ledru, L. Prenatal discrimination of a male and a female voice uttering the same sentence. Earl. Dev. Paren. 1993, 2, 217–228. [Google Scholar] [CrossRef]
- Lecanuet, J.P.; Fifer, W.P.; Krasnegor, N.A.; Smotherman, W.P. Fetal Development: A Psychobiological Perspective; Lawrance Erlbaum Associates, Inc.: New Jersey, NJ, USA, 2013. [Google Scholar]
- Mampe, B.; Friederici, A.D.; Christophe, A.; Wermke, K. Newborns’ cry melody is shaped by their native language. Curr. Biol. 2009, 19, 1994–1997. [Google Scholar] [CrossRef] [PubMed]
- DeCasper, A.J.; Fifer, W.P. Of human bonding: Newborns prefer their mothers’ voices. Science 1980, 208, 1174–1176. [Google Scholar] [CrossRef] [PubMed]
- Brazelton, T.B. The Brazelton neonatal behavior assessment scale: Introduction. Monog. Soc. Res. Chi. Dev. 1978, 43, 1–13. [Google Scholar] [CrossRef]
- Van Puyvelde, M.; Franco, F. The interaction of music and language in the ontogenesis of human communication: A multimodal parent-infant co-regulation system. In Proceedings of the International Conference on the Multimodal Experience of Music, Sheffield, UK, 23–25 March 2015. [Google Scholar]
- Kringelbach, M.L. Evidence for a caregiving instinct: Rapid differentiation of infant from adult vocalizations using magnetoencephalography. Cereb. Cor. 2016, 26, 1309–1321. [Google Scholar]
- WHO Preterm Birth. 2020. Available online: https://www.who.int/en/news–room/fact–sheets/detail/preterm–birth (accessed on 20 April 2020).
- Bliss. 2019. Prematurity Statistics in the UK. Available online: https://bliss.org.uk/research–campaigns/research/neonatal–care–statistics/prematurity–statistics–in–the–uk (accessed on 20 April 2020).
- Bliss. 2020. Available online: https://bliss.org.uk/research-campaigns/research/neonatal-care-statistics/prematurity-statistics-in-the-uk (accessed on 20 April 2020).
- Anderson, P. Neuropsychological outcomes of children born very preterm. Sem. Fet. Neon. Med. 2014, 19, 90–96. [Google Scholar] [CrossRef]
- François, C.; Rodriguez-Fornells, A.; Teixidó, M.; Agut, T.; Bosch, L. Attenuated brain responses to speech sounds in moderate preterm infants at term age. Dev. Sci. 2021, 24, e12990. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Gayraud, F. Influence of preterm birth on early lexical and grammatical acquisition. First Lang. 2007, 27, 73–159. [Google Scholar]
- Stolt, S.; Klippi, A.; Launonen, K.; Munck, P.; Lehtonen, L.; Lapinleimu, H.; Haataja, L. Size and composition of the lexicon in prematurely born very-low-birth-weight and full-term Finnish children at two years of age. J. Child. Lang. 2007, 34, 283–310. [Google Scholar] [CrossRef]
- Hirvonen, M.; Ojala, R.; Korhonen, P.; Haataja, P.; Eriksson, K.; Gissler, M.; Tammela, O. Visual and hearing impairments after preterm birth. Pediatrics 2018, 142, 2017–3888. [Google Scholar] [CrossRef]
- Kuhn, P.; Dufour, A.; Zores, C. The Auditory Sensitivity of Preterm Infants Toward their Atypical Auditory Environment in the NICU and their Attraction to Human Voices; Springer: Cham, Switzerland, 2017; pp. 113–130. [Google Scholar]
- Moon, C. Prenatal Experience with the Maternal Voice; Springer: Cham, Switzerland, 2017; pp. 25–37. [Google Scholar]
- Vohr, B.R. Language and hearing outcomes of preterm infants. Semin. Perinat. 2016, 40, 510–519. [Google Scholar] [CrossRef]
- Stipdonk, L.W.; Weisglas-Kuperus, N.; Franken, M.C.J.; Nasserinejad, K.; Dudink, J.; Goedegebure, A. Auditory brainstem maturation in normal-hearing infants born preterm: A meta-analysis. Dev. Med. Child. Neurol. 2016, 58, 1009–1015. [Google Scholar] [CrossRef]
- Zimmerman, E.; Lahav, A. Ototoxicity in preterm infants: Effects of genetics, aminoglycosides, and loud environmental noise. J. Perinat. 2013, 33, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Lahav, A.; Skoe, E. An acoustic gap between the NICU and womb: A potential risk for compromised neuroplasticity of the auditory system in preterm infants. Front. Neurosci. 2014, 8, 381. [Google Scholar] [CrossRef] [PubMed]
- White, R.D. Recommended NICU design standards and the physical environment of the NICU. J. Perinat. 2013, 33, S1. [Google Scholar] [CrossRef]
- Komlo, C.; Morriss, M.; Ries, R.; Velez, M. Cross-Sectional Analysis of Sound Levels in the Neonatal Intensive Care Unit (NICU) at Thomas Jefferson University Hospital; SKMC JeffMD Scholarly Inquiry: Philadelphia, PA, USA, 2019. [Google Scholar]
- Marik, P.E.; Fuller, C.; Levitov, A.; Moll, E. Neonatal incubators: A toxic sound environment for the preterm infant? Ped. Crit. Care Med. 2012, 13, 685–689. [Google Scholar] [CrossRef]
- Surenthiran, S.S.; Wilbraham, K.; May, J.; Chant, T.; Emmerson, A.J.B.; Newton, V.E. Noise levels within the ear and post-nasal space in neonates in intensive care. Arch. Dis. Child. Fetal Neonat. Ed. 2003, 88, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, N.; Khosravi, N.; Ranjbar, A.; Godarzi, Z.; Karimi, A. The effectiveness of earmuffs on the physiologic and behavioral stability in preterm infants. Int. J. Ped. Otorhinolar. 2017, 98, 43–47. [Google Scholar] [CrossRef]
- Morris, B.H.; Philbin, M.K.; Bose, C. Physiological effects of sound on the newborn. J. Perin. 2000, 20, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Wachman, E.M.; Lahav, A. The effects of noise on preterm infants in the NICU. Arch. Dis. Child. Fet. Neonat. Ed. 2011, 96, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, F.; Parra, J.; Berne-Audéoud, F.; Marcus, L.; Barisnikov, K.; Gentaz, E.; Debillon, T. Sound interferes with the early tactile manual abilities of preterm infants. Sci. Rep. 2016, 6, 23329. [Google Scholar] [CrossRef]
- Graven, S.N.; Browne, J.V. Auditory development in the fetus and infant. Newb. Inf. Nurs. Rev. 2008, 8, 187–193. [Google Scholar] [CrossRef]
- Huotilainen, M. A new dimension on foetal language learning. Acta Paed. 2013, 102, 102–103. [Google Scholar] [CrossRef]
- Lester, B.M.; Salisbury, A.L.; Hawes, K.; Dansereau, L.M.; Bigsby, R.; Laptook, A.; Padbury, J.F. 18-month follow-up of infants cared for in a single-family room neonatal intensive care unit. J. Ped. 2016, 177, 84–89. [Google Scholar] [CrossRef]
- Gray, L.; Philbin, M.K. Effects of the neonatal intensive care unit on auditory attention and distraction. Clin. Perinat. 2004, 31, 243–260. [Google Scholar] [CrossRef]
- Smith, S.W.; Ortmann, A.J.; Clark, W.W. Noise in the neonatal intensive care unit: A new approach to examining acoustic events. Noise Health 2018, 20, 121. [Google Scholar]
- Filippa, M.; Panza, C.; Ferrari, F.; Frassoldati, R.; Kuhn, P.; Balduzzi, S.; D’Amico, R. Systematic review of maternal voice interventions demonstrates increased stability in preterm infants. Acta Paed. 2017, 106, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Saliba, S.; Esseily, R.; Filippa, M.; Kuhn, P.; Gratier, M. Exposure to human voices has beneficial effects on preterm infants in the neonatal intensive care unit. Acta Paed. 2018, 107, 1122–1130. [Google Scholar] [CrossRef]
- Webb, A.R.; Heller, H.T.; Benson, C.B.; Lahav, A. Mother’s voice and heartbeat sounds elicit auditory plasticity in the human brain before full gestation. Proc. Nat. Acad. Sci. USA 2015, 112, 3152–3157. [Google Scholar] [CrossRef] [PubMed]
- Liszka, L.; Smith, J.; Mathur, A.; Schlaggar, B.L.; Colditz, G.; Pineda, R. Differences in early auditory exposure across neonatal environments. Early Hum. Dev. 2019, 136, 27–32. [Google Scholar] [CrossRef] [PubMed]
- LENA. Research Foundation; LENA: Boulder, CO, USA. Available online: https://www.lena.org/ (accessed on 23 April 2020).
- Fernald, A.; Weisleder, A. Twenty years after “meaningful differences”, it’s time to reframe the “deficit” debate about the importance of children’s early language experience. Hum. Dev. 2015, 58, 1. [Google Scholar] [CrossRef]
- Ramírez, N.F.; Lytle, S.R.; Kuhl, P.K. Parent coaching increases conversational turns and advances infant language development. Proc. Nat. Acad. Sci. USA 2020, 117, 3484–3491. [Google Scholar] [CrossRef]
- Romeo, R.R.; Leonard, J.A.; Robinson, S.T.; West, M.R.; Mackey, A.P.; Rowe, M.L.; Gabrieli, J.D. Beyond the 30-million-word gap: Children’s conversational exposure is associated with language-related brain function. Psych. Sci. 2018, 29, 700–710. [Google Scholar] [CrossRef]
- Romeo, R.R.; Segaran, J.; Leonard, J.A.; Robinson, S.T.; West, M.R.; Mackey, A.P.; Gabrieli, J.D. Language exposure relates to structural neural connectivity in childhood. J. Neurosci. 2018, 38, 7870–7877. [Google Scholar] [CrossRef]
- François, C.; Garcia-Alix, A.; Bosch, L.; Rodriguez-Fornells, A. Signatures of brain plasticity supporting language recovery after perinatal arterial ischemic stroke. Brain Lang. 2021, 212, 104880. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R.; Schadl, K.; Cahill-Rowley, K.; Yeom, K.; Stevenson, D.; Rose, J. Neonatal brain microstructure and machine-learning-based prediction of early language development in children born very preterm. Ped. Neuro. 2020, 108, 86–92. [Google Scholar] [CrossRef]
- Manske, R.L. Interventions to Reduce the Effects of NICU Noise in Preterm Neonates. Ph.D. Thesis, University of Central Florida, Orlando, FL, USA, 2017. [Google Scholar]
- Casavant, S.G.; Bernier, K.; Andrews, S.; Bourgoin, A. Noise in the neonatal intensive care unit: What does the evidence tell us? Adv. Neon. Care. 2017, 17, 265–273. [Google Scholar] [CrossRef]
- Almadhoob, A.; Ohlsson, A. Sound reduction management in the neonatal intensive care unit for preterm or very low birth weight infants. Cochr. Data. Syst. Rev. 2020, 1, CD010333. [Google Scholar] [CrossRef]
- Kuhn, P.; Sizun, J.; Casper, C.; GREEN Study Group from the French Neonatal Society; Allen, A.; Audeoud, F.; Zores, C. Recommendations on the environment for hospitalised newborn infants from the French neonatal society: Rationale, methods and first recommendation on neonatal intensive care unit design. Acta Paed. 2018, 107, 1860–1866. [Google Scholar] [CrossRef]
- EFCNI. European Standards of Care for Newborn Health; European Foundation for the Care of Newborn Infants: Munich, Germany, 2018. [Google Scholar]
- Laudert, S.; Liu, W.F.; Blackington, S.; Perkins, B.; Martin, S.; MacMillan–York, E.; Handyside, J. Implementing potentially better practices to support the neurodevelopment of infants in the NICU. J. Perinat. 2007, 27, S75–S93. [Google Scholar] [CrossRef] [PubMed]
- Kostilainen, K.; Mikkola, K.; Erkkilä, J.; Huotilainen, M. Effects of maternal singing during kangaroo care on maternal anxiety, wellbeing, and mother-infant relationship after preterm birth: A mixed methods study. Nord. J. Mus. Ther. 2020, 1–20. [Google Scholar] [CrossRef]
- Arya, R.; Chansoria, M.; Konanki, R.; Tiwari, D.K. Maternal music exposure during pregnancy influences neonatal behaviour: An open-label randomized controlled trial. Int. J. Pediatr. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- van der Heijden, M.J.; Oliai Araghi, S.; Jeekel, J.; Reiss, I.K.M.; Hunink, M.M.; van Dijk, M. Do hospitalized premature infants benefit from music interventions? A systematic review of randomized controlled trials. PLoS ONE 2016, 11, e0161848. [Google Scholar] [CrossRef] [PubMed]
- Loewy, J.; Stewart, K.; Dassler, A.M.; Telsey, A.; Homel, P. The effects of music therapy on vital signs, feeding, and sleep in premature infants. Pedriatics 2013, 131, 902–918. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Zores, C.; Pebayle, T.; Hoeft, A.; Langlet, C.; Escande, B.; Dufour, A. Infants born very preterm react to variations of the acoustic environment in their incubator from a minimum signal-to-noise ratio threshold of 5 to 10 dBA. Ped. Res. 2012, 71, 386–392. [Google Scholar] [CrossRef]
- Dearn, T.; Shoemark, H. The effect of maternal presence on premature infant response to recorded music. J. Obst. Gynec. Neon. Nurs. 2014, 43, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Van Dokkum, N.H.; Jaschke, A.C.; Ravensbergen, A.G.; Reijneveld, S.A.; Hakvoort, L.; De Kroon, M.L.; Bos, A.F. Feasibility of live-performed music therapy for extremely and very preterm infants in a tertiary NICU. Front. Ped. 2020, 8, 665. [Google Scholar] [CrossRef]
- Stamou, L.; Evaggelou, F.; Stamou, V.; Diamanti, E.; Loewy, J.V. The effects of live singing on the biophysiological functions of preterm infants hospitalized in a neonatal intensive care unit in Greece: A pilot study. Mus. Med. 2020, 12, 109–121. [Google Scholar] [CrossRef]
- Coombes, E.; Muzaffar, I.A. The singing unit—A pilot study investigating the efficacy of a music therapy singing intervention in a local neonatal unit to support parent/infant bonding and reduce parental anxiety. J. Neon. Nurs. 2021, 27, 47–51. [Google Scholar] [CrossRef]
- Segal, F. Mother Ship; Random House: London, UK, 2019; p. 17. [Google Scholar]
- Lindberg, B.; Öhrling, K. Experiences of having a prematurely born infant from the perspective of mothers in northern Sweden. Int. J. Circump. Health 2008, 67, 461–471. [Google Scholar] [CrossRef]
- Reid, T. Maternal identity in preterm birth. J. Child. Health Care 2000, 4, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, B.; Axelsson, K.; Öhrling, K. The birth of premature infants: Experiences from the fathers’ perspective. J. Neon. Nurs. 2007, 13, 142–149. [Google Scholar] [CrossRef]
- Olshtain-Mann, O.; Auslander, G.K. Parents of preterm infants two months after discharge from the hospital: Are they still at (parental) risk? Health Soc. Work. 2008, 33, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, F.M. Cyber security issues for Advanced Metering Infrastructure (AMI). In Proceedings of the IEEE Power and Energy Society General Meeting-Conversion and Delivery of Electrical Energy in the 21st Century, Pittsburgh, PA, USA, 20–24 July 2008. [Google Scholar]
- Boykova, M. Transition from hospital to home in parents of preterm infants: A literature review. J. Perin. Neon. Nurs. 2016, 34, 327–348. [Google Scholar] [CrossRef]
- Philips-Pula, L.; Pickler, R.; McGrath, J.; Brown, L.; Dusing, S. Caring for a preterm infant at home. J. Perin. Neon. Nurs. 2013, 27, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Feeley, N.; Zelkowitz, P.; Cormier, C.; Charbonneau, L.; Lacroix, A.; Papageorgiou, A. Posttraumatic stress among mothers of very low birthweight infants at 6 months after discharge from the neonatal intensive care unit. Appl. Nurs. Res. 2011, 24, 114–117. [Google Scholar] [CrossRef]
- Shaw, R.J.; Bernard, R.S.; Beblois, T.; Ikuta, L.M.; Ginzburg, K.; Koopman, C. The relationship between acute stress disorder and post-traumatic stress disorder in the neonatal intensive care unit. Psychosomatics 2009, 50, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Medina, I.M.F.; Granero-Molina, J.; Fernández-Sola, C.; Hernández-Padilla, J.M.; Ávila, M.C.; Rodríguez, M.D. Bonding in neonatal intensive care units: Experiences of extremely preterm infants’ mothers. Women Birth 2018, 31, 325–330. [Google Scholar] [CrossRef]
- Basner, M.; Babisch, W.; Davis, A.; Brink, M.; Clark, C.; Janssen, S.; Stansfeld, S. Auditory and non-auditory effects of noise on health. Lancet 2014, 383, 1325–1332. [Google Scholar] [CrossRef]
- Erickson, L.C.; Newman, R.S. Influences of background noise on infants and children. Curr. Dir. Psych. Sci. 2017, 26, 451–457. [Google Scholar] [CrossRef]
- Rice, T. Hearing and the Hospital: Sound, Listening, Knowledge and Experience; Sean Kingston Publishing: London, UK, 2013. [Google Scholar]
- Alkozei, A.; McMahon, E.; Lahav, A. Stress levels and depressive symptoms in NICU mothers in the early postpartum period. J. Matern. Fetal Neon. Med. 2014, 27, 1738–1743. [Google Scholar] [CrossRef]
- Feeley, N.; Robins, S.; Charbonneau, L.; Genest, C.; Lavigne, G.; Lavoie-Tremblay, M. NICU nurses’ stress and work environment in an open ward compared to a combined pod and single-family room design. Adv. Neon. Care. 2019, 19, 416–424. [Google Scholar] [CrossRef]
- Suttora, C.; Spinelli, M.; Monzani, D. From prematurity to parenting stress: The mediating role of perinatal post-traumatic stress disorder. Eur. J. Dev. Psych. 2013, 11, 478–493. [Google Scholar] [CrossRef]
- O’Donovan, A.; Nixon, E. Weathering the storm: Mothers’ and fathers’ experiences of parenting a preterm infant. Inf. Ment. Health J. 2019, 40, 573–587. [Google Scholar] [CrossRef]
- Candelori, C.; Trumello, C.; Babore, A.; Keren, M.; Romanelli, R. The experience of premature birth for fathers: The application of the clinical interview for parents of high-risk infants (CLIP) to an Italian sample. Front. Psych. 2015, 6, 1444. [Google Scholar] [CrossRef] [PubMed]
- Koliouli, F.; Zaouche-Gaudron, C.; Raynaud, J.P. Stress, coping, and post-traumatic stress disorder of French fathers of premature infants. Newborn Inf. Nurs. Rev. 2016, 16, 110–114. [Google Scholar] [CrossRef]
- Adama, E.A.; Sundin, D.; Bayes, S. Ghanaian fathers’ experiences of caring for preterm infants, a journey of exclusion. J. Neon. Nurs. 2017, 23, 275–281. [Google Scholar] [CrossRef]
- Schecter, R.; Pham, T.; Hua, A.; Spinazzola, R.; Sonnenklar, J.; Li, D.; Milanaik, R. Prevalence and longevity of PTSD symptoms among parents of NICU infants analyzed across gestational age categories. Clin. Ped. 2020, 59, 163–169. [Google Scholar] [CrossRef]
- Turpin, H.; Urben, S.; Ansermet, F.; Borghini, A.; Murray, M.M.; Müller–Nix, C. The interplay between prematurity, maternal stress and children’s intelligence quotient at age 11: A longitudinal study. Sci. Rep. 2019, 9, 1–9. [Google Scholar]
- Callahan, J.L.; Borja, S.E.; Hynan, M.T. Modification of the perinatal PTSD questionnaire to enhance clinical utility. J. Perin. 2006, 26, 533–539. [Google Scholar] [CrossRef] [PubMed]
- DeVellis, R.F. Scale Development: Theory and Applications; Sage Publications: London, UK, 2019. [Google Scholar]
- QUALTRICS. Qualtrics (Software); Qualtrics: Provo, UT, USA, 2014. [Google Scholar]
- Field, A. Discovering Statistics Using IBM SPSS Statistics, 4th ed.; Sage: London, UK, 2013. [Google Scholar]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psych. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Busse, M.; Stromgren, K.; Thorngate, L.; Thomas, K.A. Parents’ responses to stress in the neonatal intensive care unit. Crit. Care Nurse 2013, 33, 52–59. [Google Scholar] [CrossRef]
- Turan, T.; Baskale, H.; Öncel, G. Determining the psychometric properties of the Turkish version of the nurse-parent support tool and the stress levels of parents of premature infants hospitalized in the neonatal intensive care unit. Clin. Nurse Spec. 2016, 30, E1–E10. [Google Scholar] [CrossRef]
- Thomas, K.A.; Martin, P.A. NICU sound environment and the potential problems for caregivers. J. Perin. 2000, 20, S94–S99. [Google Scholar] [CrossRef]
- Santos, J.; Carvalhais, C.; Xavier, A.; Silva, M.V. Assessment and characterization of sound pressure levels in Portuguese neonatal intensive care units. Arch. Environ. Occup. Health. 2018, 73, 121–127. [Google Scholar] [CrossRef]
- Bremmer, P.; Byers, J.F.; Kiehl, E. Noise and the premature infant: Physiological effects and practice implications. J. Obst. Gynec. Neon. Nurs. 2003, 32, 447–454. [Google Scholar] [CrossRef]
- Filippa, M.; Devouche, E.; Arioni, C.; Imberty, M.; Gratier, M. Live maternal speech and singing have beneficial effects on hospitalized preterm infants. Acta Paed. 2013, 102, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Loewy, J. NICU music therapy: Song of kin as critical lullaby in research and practice. Ann. N.Y. Acad. Sci. 2015, 1337, 178–185. [Google Scholar] [CrossRef]
- Ettenberger, M.; Rojas Cárdenas, C.; Parker, M.; Odell-Miller, H. Family-centred music therapy with preterm infants and their parents in the Neonatal Intensive Care Unit (NICU) in Colombia-A mixed-methods study. Nord. J. Mus. Ther. 2017, 26, 207–234. [Google Scholar] [CrossRef]
- Filippa, M. Early Vocal Contact: Direct Talking and Singing to Preterm Infants in the NICU; Springer: Cham, Germany, 2017; pp. 133–150. [Google Scholar]
- Tarja, P.; Anne, K.; Timo, S.; Outi, P.; Helena, L. Are there differences between the expectations of parents, nurses and physicians when using music in NICU? Open J. Nurs. 2012, 2, 215–221. [Google Scholar] [CrossRef][Green Version]
- Arnon, S.; Shapsa, A.; Forman, L.; Regev, R.; Bauer, S.; Litmanovitz, I.; Dolfin, T. Live music is beneficial to preterm infants in the neonatal intensive care unit environment. Birth 2006, 33, 131–136. [Google Scholar] [CrossRef]
- Rossman, B.; Greene, M.M.; Meier, P.P. The role of peer support in the development of maternal identity for “NICU moms”. J. Obst. Gynec. Neon. Nurs. 2015, 44, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Caskey, M.; Stephens, B.; Tucker, R.; Vohr, B. Adult talk in the NICU with preterm infants and developmental outcomes. Pedriatics 2014, 133, 578–584. [Google Scholar] [CrossRef]
- Davis, L.; Mohay, H.; Edwards, H. Mothers’ involvement in caring for their premature infants: An historical overview. J. Adv. Nurs. 2003, 42, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Feeley, N.; Genest, C.; Niela-Vilén, H.; Charbonneau, L.; Axelin, A. Parents and nurses balancing parent-infant closeness and separation: A qualitative study of NICU nurses’ perceptions. BMC Ped. 2016, 16, 134. [Google Scholar] [CrossRef]
- Heidari, H.; Hasanpour, M.; Fooladi, M. The experiences of parents with infants in Neonatal Intensive Care Unit. Iran. J. Nurs. Midwif. Res. 2013, 18, 208. [Google Scholar]
- Oller, D.K.; Caskey, M.; Yoo, H.; Bene, E.R.; Jhang, Y.; Lee, C.C.; Vohr, B. Preterm and full-term infant vocalization and the origin of language. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- van Beek, Y.; Hopkins, B.; Hoeksma, J.B. Development of communicative behaviors in preterm infants: The effects of birthweight status and gestational age. Inf. Behav. Dev. 1994, 17, 107–117. [Google Scholar] [CrossRef]
- Harding, C.; Levin, A.; Crossley, S.L.; Murphy, R.; van den Engel-Hoek, L. Effects of early communication intervention on speech and communication skills of preterm infants in the neonatal intensive care unit (NICU): A systematic review. J. Neon. Nurs. 2019, 25, 177–188. [Google Scholar] [CrossRef]
- Lonstein, J.S.; Lévy, F.; Fleming, A.S. Common and divergent psychobiological mechanisms underlying maternal behaviors in non-human and human mammals. Horm. Behav. 2015, 73, 156–185. [Google Scholar] [CrossRef] [PubMed]
- LaGasse, L.L.; Neal, A.R.; Lester, B.M. Assessment of infant cry: Acoustic cry analysis and parental perception. Ment. Retard. Dev. Dis. Res. Rev. 2005, 11, 83–93. [Google Scholar] [CrossRef]
- Singer, L.T.; Fulton, S.; Kirchner, H.L.; Eisengart, S.; Lewis, B.; Short, E.; Baley, J.E. Longitudinal predictors of maternal stress and coping after very low-birth-weight birth. Arch. Ped. Adoles. Med. 2010, 164, 518–524. [Google Scholar] [CrossRef]
- McManus, B.M.; Poehlmann, J. Maternal depression and perceived social support as predictors of cognitive function trajectories during the first 3 years of life for preterm infants in Wisconsin. Child Care Health Dev. 2012, 38, 425–434. [Google Scholar] [CrossRef]
- Holditch-Davis, D.; Miles, M.S.; Burchinal, M.R.; Goldman, B.D. Maternal role attainment with medically fragile infants: Part 2. Relationship to the quality of parenting. Res. Nurs. Health 2011, 34, 35–48. [Google Scholar] [CrossRef]
- Kenner, C.; Lott, J.W. Comprehensive Neonatal Care: An Interdisciplinary Approach; Elsevier: Philadelphia, PA, USA, 2007. [Google Scholar]
- Lundqvist, P.; Weis, J.; Sivberg, B. Parents’ journey caring for a preterm infant until discharge from hospital-based neonatal home care—A challenging process to cope with. J. Clin. Nurs. 2019, 28, 2966–2978. [Google Scholar] [CrossRef]
- Ionio, C.; Lista, G.; Mascheroni, E.; Olivari, M.G.; Confalonieri, E.; Mastrangelo, M.; Scelsa, B. Premature birth: Complexities and difficulties in building the mother-child relationship. J. Repr. Inf. Psych. 2017, 35, 509–523. [Google Scholar] [CrossRef]
- Karatzias, T.; Chouliara, Z.; Maxton, F.; Freer, Y.; Power, K. Post-traumatic symptomatology in parents with premature infants: A systematic review of the literature. J. Pren. Perin. Psych. Health. 2007, 21, 249. [Google Scholar]
- Müller, M.; Tronick, E.; Zietlow, A.L.; Nonnenmacher, N.; Verschoor, S.; Traeuble, B. Effects of maternal anxiety disorders on infant self-comforting behaviors: The role of maternal bonding, infant gender and age. Psychopathology 2016, 49, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Broeder, J.L. Motherhood Too Soon: Beginning Mothering from the Neonatal Intensive Care Unit. Ph.D. Thesis, Saint Louis University, St. Louis, MO, USA, 2003. [Google Scholar]
- Fenwick, J.; Barclay, L.; Schmied, V. Craving closeness: A grounded theory analysis of women’s experiences of mothering in the Special Care Nursery. Wom. Birth. 2008, 21, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.C.; SteelFisher, G.K.; Salhi, C.; Shen, L.Y. Coping with the neonatal intensive care unit experience: Parents’ strategies and views of staff support. J. Perin. Neon. Nurs. 2012, 26, 343–352. [Google Scholar] [CrossRef]
- Arnold, L.; Sawyer, A.; Rabe, H.; Abbott, J.; Gyte, G.; Duley, L.; Ayers, S. Parents’ first moments with their very preterm babies: A qualitative study. BMJ Open 2013, 3, e002487. [Google Scholar] [CrossRef] [PubMed]
- Lasiuk, G.C.; Comeau, T.; Newburn-Cook, C. Unexpected: An interpretive description of parental traumas’ associated with preterm birth. BMC Pregn. Childbirth 2013, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Horsch, A.; Tolsa, J.F.; Gilbert, L.; Du Chêne, L.J.; Müller-Nix, C.; Graz, M.B. Improving maternal mental health following preterm birth using an expressive writing intervention: A randomized controlled trial. Child. Psych. Hum. Dev. 2016, 47, 780–791. [Google Scholar] [CrossRef]
- Cook, N.; Ayers, S.; Horsch, A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: A systematic review. J. Affect. Disord. 2018, 225, 18–31. [Google Scholar] [CrossRef]
- Kersting, A.; Dorsch, M.; Wesselmann, U.; Lüdorff, K.; Witthaut, J.; Ohrmann, P.; Arolt, V. Maternal posttraumatic stress response after the birth of a very low-birth-weight infant. J. Psychosom. Res. 2004, 57, 473–476. [Google Scholar] [CrossRef]
- Treyvaud, K. Parent and family outcomes following very preterm or very low birth weight birth: A review. Sem. Fet. Neon. Med. 2014, 19, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Holditch-Davis, D.; Miles, M.S.; Weaver, M.A.; Black, B.; Beeber, L.; Thoyre, S.; Engelke, S. Patterns of distress in African American mothers of preterm infants. J. Dev. Behav. Ped. 2009, 30, 193. [Google Scholar] [CrossRef]
- Slade, P. Towards a conceptual framework for understanding post-traumatic stress symptoms following childbirth and implications for further research. J. Psychosom. Obst. Gynec. 2006, 27, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Gondwe, K.W.; White-Traut, R.; Brandon, D.; Pan, W.; Holditch-Davis, D. The role of sociodemographic factors in maternal psychological distress and mother-preterm infant interactions. Res. Nurs. Health 2017, 40, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Christie, H.; Hamilton-Giachritsis, C.; Alves-Costa, F.; Tomlinson, M.; Halligan, S.L. The impact of parental posttraumatic stress disorder on parenting: A systematic review. Eur. J. Psychotraum. 2019, 10, 1550345. [Google Scholar] [CrossRef]
- Williamson, V.; Creswell, C.; Butler, I.; Christie, H.; Halligan, S.L. Parental experiences of supporting children with clinically significant post-traumatic distress: A qualitative study of families accessing psychological services. J. Child. Adoles. Trauma 2019, 12, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Roa, E.; Ettenberger, M. Music therapy self-care group for parents of preterm infants in the Neonatal Intensive Care Unit: A clinical pilot intervention. Medicines 2018, 5, 134. [Google Scholar] [CrossRef]
- Vianna, M.N.; Barbosa, A.P.; Carvalhaes, A.S.; Cunha, A.J.L.A. Music therapy may increase breastfeeding rates among mothers of premature newborns: A randomized controlled trial. J. Ped. 2011, 8, 206–212. [Google Scholar] [CrossRef]
- Teckenberg-Jansson, P.; Huotilainen, M.; Pölkki, T.; Lipsanen, J.; Järvenpää, A.L. Rapid effects of neonatal music therapy combined with kangaroo care on prematurely-born infants. Nord. J. Mus. Ther. 2011, 20, 22–42. [Google Scholar] [CrossRef]
- Ghetti, C.; Bieleninik, Ł.; Hysing, M.; Kvestad, I.; Assmus, J.; Romeo, R.; Gold, C. Longitudinal study of music therapy’s effectiveness for premature infants and their caregivers (LongSTEP): Protocol for an international randomised trial. BMJ Open 2019, 9, e025062. [Google Scholar] [CrossRef]
- McLean, E.; McFerran Skewes, K.; Thompson, G.A. Parents’ musical engagement with their baby in the neonatal unit to support emerging parental identity: A grounded theory study. J. Neon. Nurs. 2019, 25, 78–85. [Google Scholar] [CrossRef]
- Auger, N.; Fon Sing, M.; Park, A.L.; Lo, E.; Trempe, N.; Luo, Z.C. Preterm birth in the Inuit and First Nations populations of Québec, Canada, 1981–2008. Int. J. Circump. Health 2012, 71, 17520. [Google Scholar] [CrossRef] [PubMed]
- Gueron-Sela, N.; Atzaba-Poria, N.; Meiri, G.; Marks, K. Prematurity, ethnicity and personality: Risk for postpartum emotional distress among Bedouin-Arab and Jewish women. J. Rep. Inf. Psych. 2013, 31, 81–93. [Google Scholar] [CrossRef]
- Malin, M.; Gissler, M. Maternal care and birth outcomes among ethnic minority women in Finland. BMC Pub. Health 2009, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Neu, M.; Robinson, J. Early weeks after premature birth as experienced by Latina adolescent mothers. Am. J. Matern. Nurs. 2008, 33, 32–38. [Google Scholar] [CrossRef] [PubMed]
| M | SD | Minimum | Maximum | |
|---|---|---|---|---|
| Age of the respondents (years) | 31.78 | 5.79 | 18 | 53 |
| Gestational age at the birth of the infants (weeks) | 30.79 | 4.27 | 22 | 42 |
| Days spent in NICUs (N) | 53.03 | 48.78 | 1 | 327 |
| Infant birth weight (kg) | 1.614 | 0.839 | 0.420 | 4.490 |
| Age of child when the survey was completed (months) | 17.96 | 11.86 | 1 | 46 |
| Gender of the Child | Cause of the Birth | Categories of Weight | Categories of Prematurity | Status | Equipment Infants Were Discharged with |
|---|---|---|---|---|---|
| 199 (51.6%) Female | 90 (23.3%) Preterm rupture of membranes | 111 (28.8%) Extremely low birth weight | 113 (29.3%) Extremely preterm | 329 (85.2%) Singleton | 284 (73.6%) None |
| 187 (48.8) Male | 79 (20.5%) Preeclampsia | 84 (21.8%) Very low birth weight | 113 (34.5%) Very preterm | 51 (13.2%) Twins | 67 (18.4%) Oxygen Concentrator |
| 16 (4.1%) Elective preterm delivery | 142 (36.8%) Low birth weight | 113 (29.3%) Moderately to late preterm | 1 (0.3%) Triplets | 16 (4.1%) Feeding pump | |
| 201 (52.2%) Other * | 49 (12.7%) Normal birth weight | 27 (7.0%) Full-term | 5 (1.3%) Other | 5 (1.3%) Saturation monitor | |
| 3 (0.8%) Optiflow | |||||
| 3 (0.8%) CPAP |
| Gender | Ethnicity | Occupational Groups | Highest Level of Education Achieved | Years Spent in Education |
|---|---|---|---|---|
| 378 (97.9%) Female | 361 (93.5%) White | 111 (28.8%) Intermediate managerial/ professional/ administrative | 193 (50%) College or university | 162 (42%) 14–18 |
| 7 (1.8%) Male | 8 (2.1%) Asian/Asian British | 101 (26%) Supervisory or clerical/junior managerial | 94 (24.4%) Postgraduate (e.g., Master) | 108 (28%) Over 18 |
| 1 (0.3%) Prefer not to say | 6 (1.6%) Mixed | 48 (12.4%) Skilled manual Worker | 69 (17.9%) Higher/ secondary | 59 (15%) 12–14 |
| 3 (0.8%) Black British | 36 (9.3%) Higher managerial/ administrative | 26 (6.7%) Secondary up to 16 years of age | 40 (10.4%) 9–12 | |
| 6 (1.6%) Other | 24 (6.2%) Homemaker | 3 (0.8%) Postgraduate (e.g., doctorate) | 9 (2.3%) 6–9 | |
| 2 (0.5%) Prefer not to say | 48 (17.3%) Other | 1 (0.3%) Primary school | 8 (2.1%) Less than 6 |
| Birth Weight | NICU Soundscape | Infant Reactions | Singing-Recordings | Interacting-Bonding with Baby | Parenting Confid. | Home Environ. | |
|---|---|---|---|---|---|---|---|
| Child Age | −0.04 | 0.01 | 0.00 | −0.06 | −0.09 | −0.02 | |
| Parent Age | −0.06 | −0.05 | −0.15 ** | −0.04 | 0.08 | 0.26 ** | 0.09 |
| Birth Weight | - | −0.12 * | −0.16 ** | −0.22 ** | 0.15 ** | 0.04 | 0.01 |
| NICU Soundscape | 0.31 ** | 0.08 | 0 | −0.03 | −0.04 | ||
| Infant Reactions | 0.26 ** | 0.10 | 0.31 ** | −0.05 | |||
| Singing-Recordings | 0.31 ** | 0.29 ** | 0.16 ** | ||||
| Bonding with Baby | 0.14 ** | 0.22 ** | |||||
| Parenting Confidence | 0.10 * |
| Birth Weight | PPQ-Total Score | PPQ-Intrusiveness | PPQ-Avoidance | PPQ-Arousal | |
|---|---|---|---|---|---|
| Child Age | 0.01 | 0.03 | 0.04 | −0.02 | |
| Parent Age | −0.05 | −0.12 * | −0.12 * | −0.10 * | −0.10 * |
| Birth weight | - | 0.05 | −0.03 | −0.05 | −0.05 |
| PPQ-Total Score | - | 0.81 ** | 0.92 ** | 0.90 ** | |
| Intrusiveness | - | 0.64 ** | 0.66 ** | ||
| Avoidance | - | 0.74 ** |
| PPQ Total Score | PPQ-Intrusiveness | PPQ-Avoidance | PPQ-Arousal | |
|---|---|---|---|---|
| NICU soundscape | 0.46 ** | 0.42 ** | 0.43 ** | 0.39 ** |
| Infant Reactions | 0.12 * | 0.19 ** | 0.05 | 0.13 * |
| Singing-Recordings | 0.01 | 0.07 | −0.04 | 0.04 |
| Interacting-Bonding with Baby | 0.03 | 0.06 | −0.02 | 0.07 |
| Parenting Confidence | −0.04 | 0.00 | −0.05 | −0.05 |
| Home Environment | −0.28 ** | −0.19 ** | −0.30 ** | −0.24 ** |
| β | t | p | R2 | F | p | |
|---|---|---|---|---|---|---|
| Final model | 0.30 | 42.45 | <0.001 | |||
| NICU Soundscape | 0.45 | 10.60 | <0.001 | |||
| Home Environment | −0.28 | −6.54 | <0.001 | |||
| Bonding with Baby | 0.10 | 2.35 | <0.05 | |||
| Parent Age | −0.08 | −1.9 | =0.05 | |||
| Themes | Subthemes |
|---|---|
| Sounds of machines and various inanimate objects: What’s a “beep”? | Constant “beeping” as a symbol of the parents’ ceaseless worrying |
| The role of “beeps” as traumatic reminders | |
| Sounds from human sources | The sounds of medical staff |
| The sounds of other hospitalized families | |
| Unheard parents’ sounds: “we couldn’t vocalize!” | Parents’ feelings of transparency and incapability |
| Parents’ perplexity towards other babies’ cry | |
| The sound of music as balancing the NICU’s cacophony | Music as a supporting agent for parents and babies in the NICU |
| Music as a transitional object for families when entering their home environments |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chifa, M.; Hadar, T.; Politimou, N.; Reynolds, G.; Franco, F. The Soundscape of Neonatal Intensive Care: A Mixed-Methods Study of the Parents’ Experience. Children 2021, 8, 644. https://doi.org/10.3390/children8080644
Chifa M, Hadar T, Politimou N, Reynolds G, Franco F. The Soundscape of Neonatal Intensive Care: A Mixed-Methods Study of the Parents’ Experience. Children. 2021; 8(8):644. https://doi.org/10.3390/children8080644
Chicago/Turabian StyleChifa, Maria, Tamar Hadar, Nina Politimou, Gemma Reynolds, and Fabia Franco. 2021. "The Soundscape of Neonatal Intensive Care: A Mixed-Methods Study of the Parents’ Experience" Children 8, no. 8: 644. https://doi.org/10.3390/children8080644
APA StyleChifa, M., Hadar, T., Politimou, N., Reynolds, G., & Franco, F. (2021). The Soundscape of Neonatal Intensive Care: A Mixed-Methods Study of the Parents’ Experience. Children, 8(8), 644. https://doi.org/10.3390/children8080644