Parental Stress and Scalp Hair Cortisol in Excessively Crying Infants: A Case Control Study
Abstract
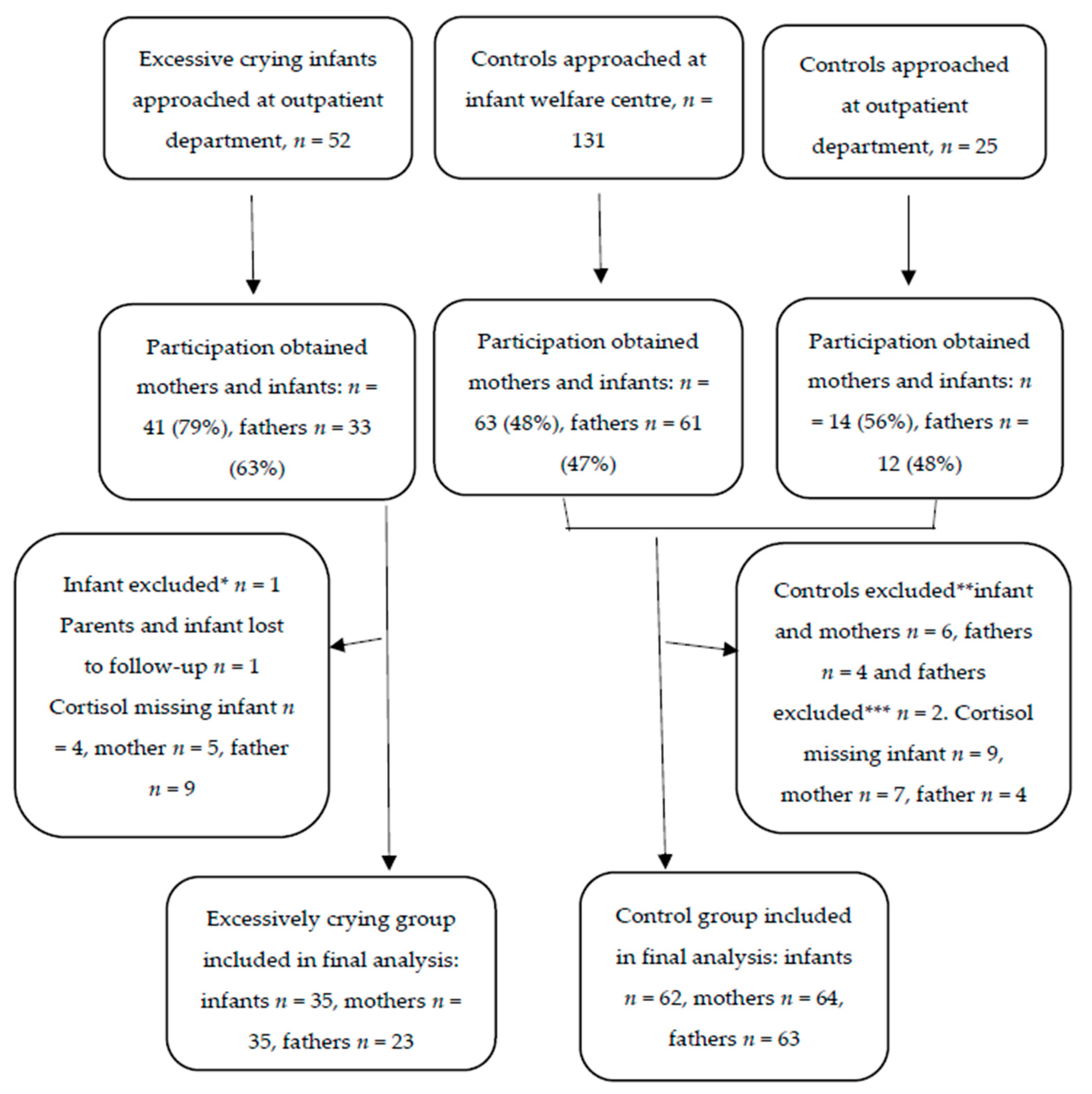
:1. Introduction
1.1. Parental Distress
1.2. HPA Axis
1.3. Scalp Hair Cortisol
- Parental HCC: Do HCC in mothers and fathers with an ECI differ from parents without an ECI (control group)?
- Parental feelings: Is parental HCC associated with experienced stress, depression, anxiety and bonding problems?
- Infant HCC: Do HCC in ECIs differ from that in control infants?
- Association between parental and infant HCC: Is there an association between parental and infant HCC and paternal and maternal HCC?
2. Materials and Methods
2.1. Study Design and Setting
2.2. Measures
2.2.1. Hair Cortisol
2.2.2. Parental Stress, Depression and Anxiety and Parent-Infant Bonding
2.2.3. Infant Crying Behavior
2.2.4. Confounders and Mediators
2.3. Statistical Analysis
3. Results
3.1. Parental HCC
3.2. Parental Feelings
3.3. Infant HCC
3.4. Association between Parental and Infant HCC
4. Discussion
4.1. Strengths and Limitations
4.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Benninga, M.A.; Faure, C.; Hyman, P.E.; Roberts, I.S.J.; Schechter, N.L.; Nurko, S. Childhood functional gastrointestinal disorders: Neonate/toddler. Gastroenterology 2016, 150, 1443–1455. [Google Scholar] [CrossRef] [PubMed]
- Halpern, R.; Coelho, R. Excessive crying in infants. J. Pediatr (Rio. J.) 2015, 92, S40–S45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott-Jupp, R. Why do babies cry? Arch. Dis. Child. 2018, 103, 1077–1079. [Google Scholar] [CrossRef]
- Cook, F.; Giallo, R.; Petrovic, Z.; Coe, A.; Seymour, M.; Cann, W.; Hiscock, H. Depression and anger in fathers of unsettled infants: A community cohort study. J. Paediatr. Child. Health 2016, 53, 131–135. [Google Scholar] [CrossRef]
- Kurth, E.; Spichiger, E.; Cignacco, E.; Kennedy, H.P.; Glanzmann, R.; Schmid, M.; Staehelin, K.; Schindler, C.; Stutz, E.Z. Predictors of Crying Problems in the Early Postpartum Period. J. Obstet. Gynecol. Neonatal Nurs. 2010, 39, 250–262. [Google Scholar] [CrossRef]
- Petzoldt, J. Systematic review on maternal depression versus anxiety in relation to excessive infant crying: It is all about the timing. Arch. Women’s Ment. Health 2017, 21, 15–30. [Google Scholar] [CrossRef]
- van den Berg, M.P.; van der Ende, J.; Crijnen, A.A.M.; Jaddoe, V.W.V.; Moll, H.A.; Mackenbach, J.P.; Hofman, A.; Hengeveld, M.W.; Tiemeier, H.; Verhulst, F.C. Paternal Depressive Symptoms During Pregnancy Are Related to Excessive Infant Crying. Pediatr. 2009, 124, e96–e103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Kruijff, I.; Veldhuis, M.S.; Tromp, E.; Vlieger, A.M.; Benninga, M.; den Berg, M.P.L. Distress in fathers of babies with infant colic. Acta Paediatr. 2021. [Google Scholar] [CrossRef]
- Stalder, T.; Steudte-Schmiedgen, S.; Alexander, N.; Klucken, T.; Vater, A.; Wichmann, S.; Kirschbaum, C.; Miller, R. Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology 2017, 77, 261–274. [Google Scholar] [CrossRef]
- Zänkert, S.; Bellingrath, S.; Wüst, S.; Kudielka, B.M. HPA axis responses to psychological challenge linking stress and disease: What do we know on sources of intra- and interindividual variability? Psychoneuroendocrinology 2019, 105, 86–97. [Google Scholar] [CrossRef]
- Brand, S.; Furlano, R.; Sidler, M.; Schulz, J.; Holsboer-Trachsler, E. ‘Oh, Baby, Please Don’t Cry!’: In Infants Suffering from Infantile Colic Hypothalamic-Pituitary-Adrenocortical Axis Activity Is Related to Poor Sleep and Increased Crying Intensity. Neuropsychobiology 2011, 64, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Brand, S.; Furlano, R.; Sidler, M.; Schulz, J.; Holsboer-Trachsler, E. Associations between Infants’ Crying, Sleep and Cortisol Secretion and Mother’s Sleep and Well-Being. Neuropsychobiology 2014, 69, 39–51. [Google Scholar] [CrossRef] [PubMed]
- White, B.P.; Gunnar, M.R.; Larson, M.C.; Donzella, B.; Barr, R.G. Behavioral and physiological responsivity, sleep, and patterns of daily cortisol production in infants with and without colic. Child. Dev. 2000, 71, 862–877. [Google Scholar] [CrossRef] [PubMed]
- Adam, E.K.; Quinn, M.E.; Tavernier, R.; McQuillan, M.; Dahlke, K.A.; Gilbert, K.E. Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology 2017, 83, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Schlotz, W. Investigating associations between momentary stress and cortisol in daily life: What have we learned so far? Psychoneuroendocrinology 2019, 105, 105–116. [Google Scholar] [CrossRef]
- Russell, E.; Koren, G.; Rieder, M.; Van Uum, S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology 2012, 37, 589–601. [Google Scholar] [CrossRef]
- Wester, V.L.; van Rossum, E.F.C. Clinical applications of cortisol measurements in hair. Eur. J. Endocrinol. 2015, 173, M1–M10. [Google Scholar] [CrossRef]
- Kalliokoski, O.; Jellestad, F.K.; Murison, R. A systematic review of studies utilizing hair glucocorticoids as a measure of stress suggests the marker is more appropriate for quantifying short-term stressors. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Staufenbiel, S.M.; Penninx, B.W.J.H.; Spijker, A.T.; Elzinga, B.M.; van Rossum, E.F.C. Hair cortisol, stress exposure, and mental health in humans: A systematic review. Psychoneuroendocrinology 2013, 38, 1220–1235. [Google Scholar] [CrossRef]
- Gray, N.; Dhana, A.; Van Der Vyver, L.; Van Wyk, J.; Khumalo, N.; Stein, D. Determinants of hair cortisol concentration in children: A systematic review. Psychoneuroendocrinology 2018, 87, 204–214. [Google Scholar] [CrossRef]
- Bates, R.; Salsberry, P.; Ford, J. Measuring Stress in Young Children Using Hair Cortisol: The State of the Science. Biol. Res. Nurs. 2017, 19, 499–510. [Google Scholar] [CrossRef]
- Baaleman, D.F.; Di Lorenzo, C.; Benninga, M.A.; Saps, M. The Effects of the Rome IV Criteria on Pediatric Gastrointestinal Practice. Curr. Gastroenterol. Rep. 2020, 22, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Noppe, G.; de Rijke, Y.B.; Dorst, K.; van den Akker, E.L.T.; van Rossum, E.F.C. LC-MS/MS-based method for long-term steroid profiling in human scalp hair. Clin. Endocrinol. (Oxf) 2015, 83, 162–166. [Google Scholar] [CrossRef] [PubMed]
- de Kruijff, I.; Noppe, G.; Kieviet, N.; Choenni, V.; Berg, M.L.-V.D.; Begijn, D.G.; Tromp, E.; Dorst, K.; van Rossum, E.F.; de Rijke, Y.B.; et al. LC-MS/MS-based reference intervals for hair cortisol in healthy children. Psychoneuroendocrinology 2020, 112, 104539. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. Source J. Health Soc. Behav. J. Health Soc. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef] [Green Version]
- Pop, V.J.; Komproe, I.H.; van Son, M.J. Characteristics of the Edinburgh post natal depression scale in The Netherlands. J. Affect. Disord. 1992, 26, 105–110. [Google Scholar] [CrossRef]
- Massoudi, P.; Hwang, C.P.; Wickberg, B. How well does the Edinburgh Postnatal Depression Scale identify depression and anxiety in fathers? A validation study in a population based Swedish sample. J. Affect. Disord. 2013, 149, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Matthey, S.; Barnett, B.; Kavanagh, D.; Howie, P. Validation of the Edinburgh Postnatal Depression Scale for men, and comparison of item endorsement with their partners. J. Affect. Disord. 2001, 64, 175–184. [Google Scholar] [CrossRef]
- Marteau, T.M.; Bekker, H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br. J. Clin. Psychol. 1992, 31, 301–306. [Google Scholar] [CrossRef]
- Spielberger, C.D. Assessment of state and trait anxiety: Conceptual and methodological issues. South. Psychol. 1985, 2, 6–16. [Google Scholar]
- van der Bij, A.K.; de Weerd, S.; Cikot, R.J.; Steegers, E.A.; Braspenning, J.C. Validation of the Dutch Short Form of the State Scale of the Spielberger State-Trait Anxiety Inventory: Considerations for Usage in Screening Outcomes. Public Health Genom. 2003, 6, 84–87. [Google Scholar] [CrossRef]
- Brockington, I.F.; Fraser, C.; Wilson, D. The Postpartum Bonding Questionnaire: A validation. Arch. Women’s Ment. Health 2006, 9, 233–242. [Google Scholar] [CrossRef]
- Brockington, I.F.; Oates, J.; George, S.; Turner, D.; Vostanis, P.; Sullivan, M.; Loh, C.; Murdoch, C. A Screening Questionnaire for mother-infant bonding disorders. Arch. Women’s Ment. Health 2001, 3, 133–140. [Google Scholar] [CrossRef]
- van Bussel, J.C.H.; Spitz, B.; Demyttenaere, K. Three self-report questionnaires of the early mother-to-infant bond: Reliability and validity of the Dutch version of the MPAS, PBQ and MIBS. Arch. Women’s Ment. Health 2010, 13, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Barr, R.G.; Kramer, M.S.; Boisjoly, C.; McVey-White, L.; Pless, I.B. Parental diary of infant cry and fuss behaviour. Arch. Dis. Child. 1988, 63, 380–387. [Google Scholar] [CrossRef] [Green Version]
- Abell, J.G.; Stalder, T.; Ferrie, J.E.; Shipley, M.J.; Kirschbaum, C.; Kivimäki, M.; Kumari, M. Assessing cortisol from hair samples in a large observational cohort: The Whitehall II study. Psychoneuroendocrinology 2016, 73, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Sauvé, B.; Koren, G.; Walsh, G.; Tokmakejian, S.; van Uum, S.H. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin. Investig. Med. 2007, 30, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Staufenbiel, S.M.; Penninx, B.W.; de Rijke, Y.B.; Akker, E.L.V.D.; van Rossum, E.F. Determinants of hair cortisol and hair cortisone concentrations in adults. Psychoneuroendocrinology 2015, 60, 182–194. [Google Scholar] [CrossRef]
- Mustonen, P.; Karlsson, L.; Scheinin, N.M.; Kortesluoma, S.; Coimbra, B.; Rodrigues, A.J.; Karlsson, H. Hair cortisol concentration (HCC) as a measure for prenatal psychological distress—A systematic review. Psychoneuroendocrinology 2018, 92, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Steudte-Schmiedgen, S.; Kirschbaum, C.; Alexander, N.; Stalder, T. An integrative model linking traumatization, cortisol dysregulation and posttraumatic stress disorder: Insight from recent hair cortisol findings. Neurosci Biobehav Rev. 2016, 69, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Zorn, J.V.; Schür, R.R.; Boks, M.P.; Kahn, R.S.; Joëls, M.; Vinkers, C.H. Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology 2017, 77, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.E.; Bosquet, E.M.; Plamondon, A.L.-R.K. The association between adversity and hair cortisol levels in humans: A meta-analysis. Psychoneuroendocrinology 2019, 103, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.; Newport, D.J.; Bonsall, R.; Miller, A.H.; Nemeroff, C.B. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am. J. Psychiatry 2001, 158, 575–581. [Google Scholar] [CrossRef]
- Dauegaard, S.; Olsen, N.J.; Heitmann, B.L.; Larsen, S.C. Familial associations in hair cortisol concentration: A cross-sectional analysis based on the Healthy Start study. Psychoneuroendocrinology 2020, 121, 104836. [Google Scholar] [CrossRef]
- Kieviet, N.; de Groot, S.; Noppe, G.; de Rijke, Y.B.; van Rossum, E.F.C.; van den Akker, E.L.T.; Dolman, K.M.; Honig, A. Is poor neonatal adaptation after exposure to antidepressant medication related to fetal cortisol levels? An explorative study. Early Hum. Dev. 2016, 98, 37–43. [Google Scholar] [CrossRef]
- Bryson, H.E.; Price, A.M.; Goldfeld, S.; Mensah, F. Associations between social adversity and young children’s hair cortisol: A systematic review. Psychoneuroendocrinology 2021, 127, 105176. [Google Scholar] [CrossRef]
- Hollanders, J.J.; van der Voorn, B.; Kieviet, N.; Dolman, K.M.; de Rijke, Y.B.; van den Akker, E.L.T.; Rotteveel, J.; Honig, A.; Finken, M.J.J. Interpretation of glucocorticoids in neonatal hair: A reflection of intrauterine glucocorticoid regulation? Endocr. Connect. 2017, 6, 692–699. [Google Scholar] [CrossRef] [Green Version]
- Romero-gonzalez, B.; Caparros-gonzalez, R.A.; Gonzalez-perez, R.; Delgado-Puertas, P.; Peralta-Ramirez, M.I. Newborn infants’ hair cortisol levels reflect chronic maternal stress during pregnancy. PLoS ONE 2018, 13, e0200279. [Google Scholar] [CrossRef] [PubMed]
- Broeks, C.W.; Choenni, V.; Kok, R.; van der Voorn, B.; de Kruijff, I.; van den Akker, E.L.T.; van Rossum, E.F.; Hoogendijk, W.J.; Hillegers, M.H.; Kamperman, A.M.; et al. An exploratory study of perinatal hair cortisol concentrations in mother–infant dyads with severe psychiatric disorders versus healthy controls. BJPsych Open 2021, 7, e28. [Google Scholar] [CrossRef]
| Excessive Crying Mean (SD) or % and (N) | Control Mean (SD) or % and (N) | p-Value | |
|---|---|---|---|
| Infants’ age (w) | 8.54 (3.37) (35) | 10.19 (3.95) (62) | 0.040 |
| Male gender | 57.1 (20/35) | 45.2 (28/62) | |
| Gestational age at birth (w) | 38.9 (1.3) (35) | 39.3 (1.4) (62) | 0.257 |
| Birthweight (g) | 3312 (534) (35) | 3433 (555) (62) | 0.150 |
| Feeding status | 0.300 | ||
| 20.0 (7/35) | 46.8 (29/62) | |
| 80.0 (28/35) | 40.3 (25/62) | |
| 0 | 12.9 (8/62) | <0.001 |
| Use of medication infant | 31.4 (11/35) | 0 (0/62) | <0.001 |
| |||
Use of medication mother
| 14.13 (5/35) | 3.1 (2/64) | 0.093 |
| Age (y) | |||
| 31.2 (3.2) (35) | 31.9 (4.6)(64) | 0.354 |
| 33.4 (4.6) (23) | 34.5 (4.9)(63) | 0.344 |
| Ethnicity (Dutch-Caucasian) | |||
| 100 (35/35) | 90.6 (58/64) | 0.087 |
| 87 (20/23) | 93.7 (59/63) | 0.378 |
| Educational level of mother | |||
| 5.7 (2/35) | 4.7 (3/64) | |
| 48.6 (17/35) | 17.2 (11/64) | 0.004 |
| 28.6 (10/35) | 43.8 (28/64) | |
| 17.1 (6/35) | 34.4 (22/64) | |
| Educational level of father | |||
| 21.7 (5/23) | 9.5 (6/63) | |
| 30.4 (7/23) | 33.3 (21/63) | 0.298 |
| 26.1 (6/23) | 30.2 (19/63) | |
| 21.7 (5/23) | 27.0 (17/63) | |
| Current Smoking | |||
| 14.7 (5/34) | 4.8 (3/63) | 0.124 |
| 17.4 (4/23) | 15.9 (10/63) | 1.000 |
| Emotional/psychiatric problems pregnancy | |||
| - Mother | 25.7 (9/35) | 10.9 (7/64) | 0.056 |
| - Father | 8.7 (2/23) | 1.6 (1/63) | 0.173 |
| Current psychiatric treatment | |||
| - Mother | 14.3 (5/35 1) | 3.1(2/64) | 0.093 |
| - Father | 8.7 (2/23) | 1.6 (1/63) | 0.173 |
| Experienced stressful events | |||
| - Mother | 54.3 (19/35) | 38.1 (24/63) | 0.122 |
| - Father | 52.2 (12/23) | 41.0 (26/63) | 0.367 |
| Negative experience of pregnancy | |||
| - Mother | 14.3 (5/35) | 6.5 (4/62) | 0.277 |
| - Father | 8.7 (2/23) | 1.6 (1/61) | 0.181 |
| Negative experience of delivery | |||
| - Mother | 14.7 (5/34) | 14.5 (9/62) | 1.000 |
| - Father | 17.4 (4/23) | 3.3 (2/61) | 0.045 |
| Variable | Excessive Crying Mean (SD) and (N) | Control Mean(SD) and (N) | p-Value |
|---|---|---|---|
| Stress (PSS) | |||
| 25.2 (8.1) (33) | 14.1 (6.9) (64) | <0.001 |
| 21.0 (6.3) (20) | 16.2 (6.1) (62) | 0.003 |
| Depression (EPDS) | |||
| 8.8 (5.2) (34) | 3.8 (3.2) (64) | <0.001 |
| 5.4 (4.3) (20) | 2.8 (2.9) (62) | 0.016 |
| State Anxiety (STAI) | |||
| 45.9 (12.3) (34) | 31.6 (9.3) (64) | <0.001 |
| 42.1 (10.0) (21) | 31.6 (7.5) (62) | <0.001 |
| Bonding behaviour (PBQ) | |||
| 15.2 (8.2) (16) | 4.8 (4.4) (64) | <0.001 |
| 18.0 (8.4) (10) | 7.9 (5.9) (62) | <0.001 |
| Crying duration (min) | 112 (78) (31) | ||
| 74 (53) (31) | 26 (24) (61) | <0.001 |
| 14 (15) (61) | <0.001 | |
| Intensity of crying | 5.9 (1.9) (13) | 2.6 (1.6) (61) | <0.001 |
| Volume of crying | 6.0 (1.8) (13) | 3.5 (5.3) (61) | 0.096 |
| Characteristics | Unadjusted Mean (95% CI) | Log Beta | p-Value | Log Adjusted Beta | p-Value | Log Adjusted Beta | p-Value |
|---|---|---|---|---|---|---|---|
| HCC infant (pg/mg) | |||||||
| Control (n = 62) | 34.0 (26.3–44.0) | Ref | Ref * | ||||
| Exc crying (n = 35) | 32.1 (25.1–40.9) | −0.06 | 0.762 | −0.14 | 0.510 | ||
| HCC mother (pg/mg) | |||||||
| Control (n = 64) | 3.2 (3.0–3.7) | Ref | Ref ** | Ref ** | |||
| Exc crying(n = 35) | 2.3 (1.8–2.9) | −0.35 | 0.009 | −0.41 | 0.002 | −0.40 | 0.003 |
| HCC father (pg/mg) | |||||||
| Control (n = 63) | 2.9 (2.5–3.5) | Ref | Ref ** | Ref *** | |||
| Exc crying (n = 23) | 1.6 (1.3—2.0) | −0.60 | <0.001 | −0.62 | <0.001 | −0.60 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Kruijff, I.; Tromp, E.; Lambregtse-van den Berg, M.P.; Vlieger, A.M.; Benninga, M.A.; de Rijke, Y.B.; van den Akker, E.L. Parental Stress and Scalp Hair Cortisol in Excessively Crying Infants: A Case Control Study. Children 2021, 8, 662. https://doi.org/10.3390/children8080662
de Kruijff I, Tromp E, Lambregtse-van den Berg MP, Vlieger AM, Benninga MA, de Rijke YB, van den Akker EL. Parental Stress and Scalp Hair Cortisol in Excessively Crying Infants: A Case Control Study. Children. 2021; 8(8):662. https://doi.org/10.3390/children8080662
Chicago/Turabian Stylede Kruijff, Ineke, Ellen Tromp, Mijke P. Lambregtse-van den Berg, Arine M. Vlieger, Marc A. Benninga, Yolanda B. de Rijke, and Erica LT. van den Akker. 2021. "Parental Stress and Scalp Hair Cortisol in Excessively Crying Infants: A Case Control Study" Children 8, no. 8: 662. https://doi.org/10.3390/children8080662
APA Stylede Kruijff, I., Tromp, E., Lambregtse-van den Berg, M. P., Vlieger, A. M., Benninga, M. A., de Rijke, Y. B., & van den Akker, E. L. (2021). Parental Stress and Scalp Hair Cortisol in Excessively Crying Infants: A Case Control Study. Children, 8(8), 662. https://doi.org/10.3390/children8080662







