Interaction between Autonomic Regulation, Adiposity Indexes and Metabolic Profile in Children and Adolescents with Overweight and Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of the Patients
2.2. Anthropometric Data
2.3. Metabolic Evaluation
2.4. Endothelium-Dependent Vasodilation: RHI
2.5. Ultrasound
2.6. Autonomic Evaluation
2.7. Statistics
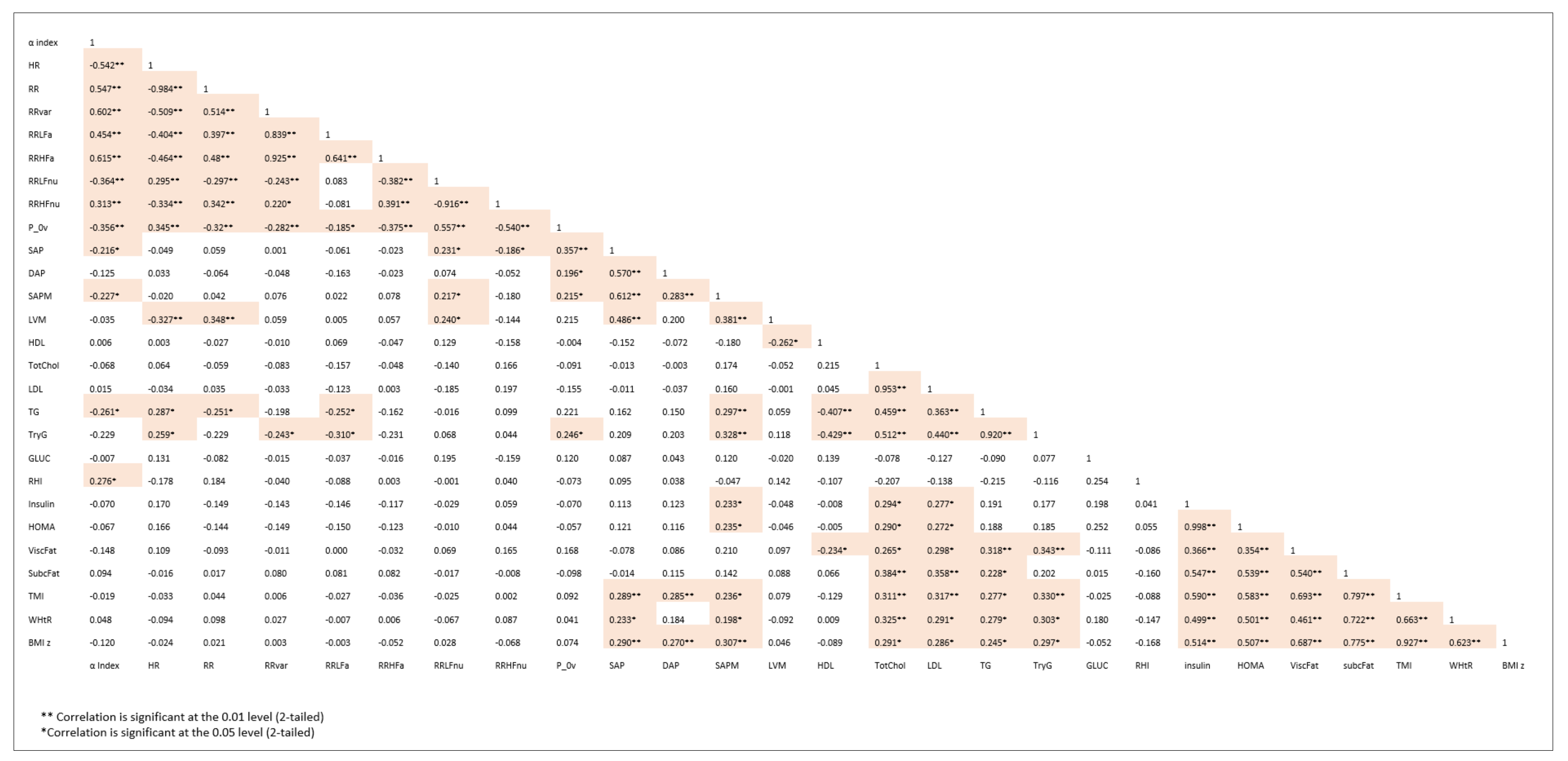
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Noncommunicable diseases: Childhood overweight and obesity. Available online: https://www.who.int/dietphysicalactivity/childhood/en/ (accessed on 28 July 2021).
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Lauria, L.; Spinelli, A.; Buoncristiano, M.; Nardone, P. Decline of childhood overweight and obesity in Italy from 2008 to 2016: Results from 5 rounds of the population-based surveillance system. BMC Public Health 2019, 19, 618. [Google Scholar] [CrossRef]
- Després, J.P. Body fat distribution and risk of cardiovascular disease: An update. Circulation 2012, 126, 1301–1313. [Google Scholar] [CrossRef]
- Vizzuso, S.; Del Torto, A.; Dilillo, D.; Calcaterra, V.; Di Profio, E.; Leone, A.; Gilardini, L.; Bertoli, S.; Battezzati, A.; Zuccotti, G.V.; et al. Visceral Adiposity Index (VAI) in Children and Adolescents with Obesity: No Association with Daily Energy Intake but Promising Tool to Identify Metabolic Syndrome (MetS). Nutrients 2021, 13, 413. [Google Scholar] [CrossRef]
- Leone, A.; Vizzuso, S.; Brambilla, P.; Mameli, C.; Ravella, S.; De Amicis, R.; Battezzati, A.; Zuccotti, G.; Bertoli, S.; Verduci, E. Evaluation of Different Adiposity Indices and Association with Metabolic Syndrome Risk in Obese Children: Is there a Winner? Int. J. Mol. Sci. 2020, 21, 4083. [Google Scholar] [CrossRef] [PubMed]
- Malavazos, A.E.; Capitanio, G.; Milani, V.; Ambrogi, F.M.; Matelloni, I.A.; Basilico, S.; Dubini, C.; Sironi, F.M.; Stella, E.; Castaldi, S.; et al. Tri-Ponderal Mass Index vs body Mass Index in discriminating central obesity and hypertension in adolescents with overweight. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Licenziati, M.R.; Iannuzzo, G.; Morlino, D.; Campana, G.; Renis, M.; Iannuzzi, A.; Valerio, G. Fat mass and vascular health in overweight/obese children. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Angoorani, H.; Karimi, Z.; Naderi, F.; Mazaherinezhad, A. Is ultrasound-measured abdominal fat thickness a reliable method for predicting metabolic diseases in obese and overweight women? Med. J. Islam. Repub. Iran. 2018, 28, 32–78. [Google Scholar] [CrossRef]
- Liao, D.; Rodríguez-Colón, S.M.; He, F.; Bixler, E.O. Childhood obesity and autonomic dysfunction: Risk for cardiac morbidity and mortality. Curr. Treat. Options Cardiovasc. Med. 2014, 16, 342. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lucini, D.; de Giacomi, G.; Tosi, F.; Malacarne, M.; Respizzi, S.; Pagani, M. Altered cardiovascular autonomic regulation in overweight children engaged in regular physical activity. Heart 2013, 99, 376–381. [Google Scholar] [CrossRef]
- Calcaterra, V.; Vandoni, M.; Correale, L.; Larizza, D.; DeBarbieri, G.; Albertini, R.; Tinelli, C.; Arpesella, M.; Bernardi, L. Deep breathing acutely improves arterial dysfunction in obese children: Evidence of functional impairment? Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1301–1309. [Google Scholar] [CrossRef]
- Calcaterra, V.; Vandoni, M.; Debarbieri, G.; Larizza, D.; Albertini, R.; Arpesella, M.; Bernardi, L. Deep breathing improves blunted baroreflex sensitivity in obese children and adolescents with insulin resistance. Int. J. Cardiol. 2012, 168, 1614–1615. [Google Scholar] [CrossRef]
- Lucini, D.; Zuccotti, G.V.; Scaramuzza, A.; Malacarne, M.; Gervasi, F.; Pagani, M. Exercise might improve cardiovascular autonomic regulation in adolescents with type 1 diabetes. Acta Diabetol. 2013, 50, 341–349. [Google Scholar] [CrossRef]
- Lucini, D.; Malacarne, M.; Gatzemeier, W.; Pagani, M. A simple home-based lifestyle intervention program to improve cardiac autonomic regulation in patients with increased cardiometabolic risk. Sustainability 2020, 12, 7671. [Google Scholar] [CrossRef]
- Brasil, I.; Monteiro, W.; Lima, T.; Seabra, A.; Farinatti, P. Effects of judo training upon body composition, autonomic function, and cardiorespiratory fitness in overweight or obese children aged 8–13 years. J. Sports Sci. 2020, 38, 2508–2516. [Google Scholar] [CrossRef]
- Speer, K.E.; Naumovski, N.; Semple, S.; McKune, A.J. Lifestyle Modification for Enhancing Autonomic Cardiac Regulation in Children: The Role of Exercise. Children 2019, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.; Kliszczewicz, B.; Vanderlei, F.M.; Monteiro, L.; Martiniano, E.C.; de Moraes, Y.M.; Mangueira, L.B.; Alcantara, G.C.; da Silva, J.; Benjamim, C.; et al. Autonomic responses induced by aerobic submaximal exercise in obese and overweight adolescents. Cardiol. Young 2019, 29, 169–173. [Google Scholar] [CrossRef]
- Solaro, N.; Pagani, M.; Lucini, D. Altered Cardiac Autonomic Regulation in Overweight and Obese Subjects: The Role of Age-and-Gender-Adjusted Statistical Indicators of Heart Rate Variability and Cardiac Baroreflex. Front. Physiol. 2021, 11, 567312. [Google Scholar] [CrossRef]
- Lucini, D.; Solaro, N.; Pagani, M. Autonomic Differentiation Map: A Novel Statistical Tool for Interpretation of Heart Rate Variability. Front. Physiol. 2018, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; De Giuseppe, R.; Biino, G.; Mantelli, M.; Marchini, S.; Bendotti, G.; Madè, A.; Avanzini, M.A.; Montalbano, C.; Cossellu, G.; et al. Relation between circulating oxidized-LDL and metabolic syndrome in children with obesity: The role of hypertriglyceridemic waist phenotype. J. Pediatric Endocrinol. Metab. 2017, 30, 1257–1263. [Google Scholar] [CrossRef]
- World Health Organization. BMI-for-age (5-19 years). Available online: https://www.who.int/tools/growth-reference-data-for-5to19-years/indicators/bmi-for-age (accessed on 28 July 2021).
- Mameli, C.; Krakauer, N.Y.; Krakauer, J.C.; Bosetti, A.; Ferrari, C.M.; Moiana, N.; Schneider, L.; Borsani, B.; Genoni, T.; Zuccotti, G. The association between a body shape index and cardiovascular risk in overweight and obese children and adolescents. PLoS ONE 2018, 13, e0190426. [Google Scholar] [CrossRef]
- Hudda, M.T.; Fewtrell, M.S.; Haroun, D.; Lum, S.; Williams, J.E.; Wells, J.; Riley, R.D.; Owen, C.G.; Cook, D.G.; Rudnicka, A.R.; et al. Development and validation of a prediction model for fat mass in children and adolescents: Meta-analysis using individual participant data. BMJ 2019, 366, l4293. [Google Scholar] [CrossRef]
- Del Ry, S.; Cabiati, M.; Bianchi, V.; Caponi, L.; Maltinti, M.; Caselli, C.; Kozakova, M.; Palombo, C.; Morizzo, C.; Marchetti, S.; et al. C-type natriuretic peptide is closely associated to obesity in Caucasian adolescents. Clin. Chim. Acta Int. J. Clin. Chem. 2016, 460, 172–177. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. The Product of Fasting Glucose and Triglycerides as Surrogate for Identifying Insulin Resistance in Apparently Healthy Subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Haller, M.J.; Stein, J.; Shuster, J.; Theriaque, D.; Silverstein, J.; Schatz, D.A.; Earing, M.G.; Lerman, A.; Mahmud, F.H. Peripheral artery tonometry demonstrates altered endothelial function in children with type 1 diabetes. Pediatr Diabetes 2007, 8, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Palombo, C.; Kozakova, M.; Morizzo, C.; Gnesi, L.; Barsotti, M.C.; Spontoni, P.; Massart, F.; Salvi, P.; Balbarini, A.; Saggese, G.; et al. Circulating endothelial progenitor cells and large artery structure and function in young subjects with uncomplicated type 1 diabetes. Cardiovasc. Diabetol. 2011, 10, 88. [Google Scholar] [CrossRef]
- Sabir, N.; Pakdemirli, E.; Sermez, Y.; Zencir, M.; Kazil, S. Sonographic assessment of changes in thickness of different abdominal fat layers in response to diet in obese women. J. Clin. Ultrasound JCU 2013, 31, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Filho, F.F.; Faria, A.N.; Kohlmann, O., Jr.; Ajzen, S.; Ribeiro, A.B.; Zanella, M.T.; Ferreira, S.R. Ultrasonography for the evaluation of visceral fat and cardiovascular risk. Hypertension 2001, 38, 713–717. [Google Scholar] [CrossRef]
- Pagani, M.; Somers, V.; Furlan, R.; Orto, S.D.; Conway, J.; Baselli, G.; Cerutti, S.; Sleight, P.; Malliani, A. Changes in Autonomic Regulation Induced by Physical Training in Mild Hypertension. Hypertension 1988, 12, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Hess, W.R. The Central Control of the Activity of Internal Organs. In Nobel Lectures, Physiology or Medicine 1942-1962; Elsevier: Amsterdam, The Netherlands, 1949; Available online: https://www.nobelprize.org/prizes/medicine/1949/hess/lecture/ (accessed on 28 July 2021).
- Ahn, A.C.; Tewari, M.; Poon, C.S.; Phillips, R.S. The clinical applications of a systems approach. PLoS Med. 2006, 3, 0956–0960. [Google Scholar] [CrossRef] [PubMed]
- Sassi, R.; Cerutti, S.; Lombardi, F.; Malik, M.; Huikuri, H.V.; Peng, C.K.; Schimdt, G.; Yamamoto, Y. Advances in heart rate variability signal analysis: Joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. Europace 2015, 17, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Pagani, M.; Montano, N.; Porta, A.; Malliani, A.; Abboud, F.M.; Birkett, C.; Somers, V.K. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation 1997, 95, 1441–1448. [Google Scholar] [CrossRef]
- Kerkhof, P.L.M.; Peace, R.A.; Handly, N. Ratiology and a Complementary Class of Metrics for Cardiovascular Investigations. Physiology 2019, 34, 250–263. [Google Scholar] [CrossRef]
- Allen, L.N.; Feigl, A.B. Reframing non-communicable diseases as socially transmitted conditions. Lancet. Glob. Health 2017, 5, e644–e646. [Google Scholar] [CrossRef][Green Version]
- Valerio, G.; Maffeis, C.; Saggese, G.; Ambruzzi, M.A.; Balsamo, A.; Bellone, S.; Bergamini, M.; Bernasconi, S.; Bona, G.; Calcaterra, V.; et al. Diagnosis, treatment and prevention of pediatric obesity: Consensus position statement of the Italian Society for Pediatric Endocrinology and Diabetology and the Italian Society of Pediatrics. Ital. J. Pediatrics 2018, 44, 88. [Google Scholar] [CrossRef]
- Michael, J.; Joyner, M.J.; Green, D.J. Exercise protects the cardiovascular system: Effects beyond traditional risk factors. J. Physiol. 2009, 587, 5551–5558. [Google Scholar]
- Kozakova, M.; Morizzo, C.; Bianchi, V.; Marchetti, S.; Federico, G.; Palombo, C. Hemodynamic overload and intra-abdominal adiposity in obese children: Relationships with cardiovascular structure and function. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Malliani, A.; Pagani, M.; Lombardi, F.; Cerutti, S. Cardiovascular neural regulation explored in the frequency domain. Circulation 1991, 84, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Kanazawa, H.; Aizawa, Y.; Ardell, J.L.; Shivkumar, K. Cardiac Innervation and Sudden Cardiac Death. Circ Res. 2015, 116, 2005–2019. [Google Scholar] [CrossRef]
- Sun, M.K. Central neural organization and control of sympathetic nervous system in mammals. Prog. Neurobiol. 1995, 47, 157–233. [Google Scholar] [CrossRef]
- Malliani, A.; Pagani, M.; Furlan, R.; Guzzetti, S.; Lucini, D.; Montano, N.; Cerutti, S.; Mela, G.S. Individual recognition by heart rate variability of two different autonomic profiles related to posture. Circulation 1997, 96, 4143–4145. [Google Scholar] [CrossRef]
- Lucini, D.; Mela, G.S.; Malliani, A.; Pagani, M. Impairment in cardiac autonomic regulation preceding arterial hypertension in humans: Insights from spectral analysis of beat-by-beat cardiovascular variability. Circulation 2002, 106, 2673–2679. [Google Scholar] [CrossRef] [PubMed]
- Lucini, D.; Cusumano, G.; Bellia, A.; Kozakova, M.; DiFede, G.; Lauro, R.; Linosa Study Group. Is reduced baroreflex gain a component of the metabolic syndrome? Insights from the LINOSA study. J. Hypertens. 2006, 24, 361–370. [Google Scholar] [CrossRef]
- Lucini, D.; Zuccotti, G.; Malacarne, M.; Scaramuzza, A.; Riboni, S.; Palombo, C.; Pagani, M. Early progression of the autonomic dysfunction observed in pediatric type 1 diabetes mellitus. Hypertension 2009, 54, 987–994. [Google Scholar] [CrossRef][Green Version]
- Wühl, E. Hypertension in childhood obesity. Acta Paediatr. 2019, 108, 37–43. [Google Scholar] [CrossRef]
- Zhou, M.S.; Wang, A.; Yu, H. Link between insulin resistance and hypertension: What is the evidence from evolutionary biology? Diabetol. Metab. Syndr. 2014, 6, 12. [Google Scholar] [CrossRef]
- Mancusi, C.; Izzo, R.; di Gioia, G.; Losi, M.A.; Barbato, E.; Morisco, C. Insulin Resistance the Hinge Between Hypertension and Type 2 Diabetes. High Blood Press. Cardiovasc. Prev. 2020, 27, 515–526. [Google Scholar] [CrossRef]
- Curtis, B.M.; O’Keefe, J.H. Autonomic tone as a cardiovascular risk factor: The dangers of chronic fight or flight. Mayo. Clin. Proc. 2002, 77, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Forkert, E.C.O.; Rendo-Urteaga, T.; Nascimento-Ferreira, M.V.; Ferreira de Moraes, A.C.; Moreno, L.A.; Barbosa de Carvalho, H. Abdominal obesity and cardiometabolic risk in children and adolescents, are we aware of their relevance? Nutrire 2016, 41, 15. [Google Scholar] [CrossRef]
- James Fisher, J.P.; Fadel, P.J. Therapeutic strategies for targeting excessive central sympathetic activation in human hypertension. Exp. Physiol. 2010, 95, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Solaro, N.; Lucini, D.; Pagani, M. Handling missing data in observational clinical studies concerning cardiovascular risk: An insight into critical aspects. In Data Science–Innovative Developments in Data Analysis and Clustering; Series on Studies in Classification, Data Analysis, and Knowledge Organization; Palumbo, F., Montanari, A., Vichi, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 131–144. ISBN 978-3-319-55722-9. [Google Scholar]

| Variables | NW | OW/OB | Significance |
|---|---|---|---|
| Height (cm) | 158.9 ± 12.2 | 157.6 ± 11.6 | 0.549 |
| Weight (kg) | 48.9 ± 11.3 | 72.3 ± 18.7 | <0.001 |
| BMI (kg/m2) | 19.07 ± 2.04 | 28.8 ± 5.51 | <0.001 |
| BMI z score (-) | 0.11 ± 0.65 | 2.75 ± 1.09 | <0.001 |
| Triponderal Mass Index (kg/m3) | 12.01 ± 0.95 | 18.34 ± 3.56 | <0.001 |
| Waist circumference (cm) | 72.0 ± 10.1 | 94.2 ± 14.9 | <0.001 |
| WHtR (-) | 0.41 ± 0.14 | 0.59 ± 0.11 | <0.001 |
| Visceral fat (cm) | 2.14 ± 0.71 | 4.08 ± 1.31 | <0.001 |
| Subcutaneous fat (cm) | 1.03 ± 0.51 | 3.49 ± 0.98 | <0.001 |
| SAP (mmHg) | 107.4 ± 11.6 | 113.7 ± 10.2 | 0.003 |
| DAP (mmHg) | 63.8 ± 6.6 | 66.5 ± 6.3 | 0.032 |
| SAP (percentile) | 43.9 ± 26.9 | 62.6 ± 29.2 | <0.001 |
| DAP (percentile) | 48.5 ± 19.3 | 58.2 ± 17.3 | 0.006 |
| HR (b/min) | 71.8 ± 9.5 | 70.0 ± 9.9 | 0.324 |
| LV mass (g) | 119.4 ± 43.0 | 122.9 ± 36.0 | 0.716 |
| RHI (-) | 1.86 ± 0.48 | 1.59 ± 0.44 | 0.037 |
| Total cholesterol (mg/dL) | 130.5 ± 39.7 | 165.8 ± 35.9 | 0.001 |
| LDL cholesterol (mg/dL) | 73.4 ± 32.8 | 100.4 ± 30.8 | 0.003 |
| HDL cholesterol (mg/dL) | 46.8 ± 12.5 | 47.3 ± 12.2 | 0.868 |
| Triglycerides (mg/dL) | 51.5 ± 20.0 | 89.8 ± 68.0 | 0.030 |
| TryGI (mg/dL)2 | 7.62 ± 0.44 | 7.96 ± 0.58 | 0.145 |
| Fasting glucose (mg/dL) | 78.5 ± 7.7 | 79.0 ± 11.0 | 0.935 |
| Insulin (pmol/l) | 48.0 ± 18.4 | 128.7 ± 83.6 | <0.001 |
| HbA1c (mmol/mol) | 5.16 ± 0.28 | 5.43 ± 0.31 | 0.001 |
| HOMA-iR | 0.89 ± 0.31 | 2.25 ± 1.41 | <0.001 |
| Variables | NW | OW/OB | Significance |
|---|---|---|---|
| Alpha index (ms/mmHg) | 28.0 ± 19.0 | 24.2 ± 13.2 | 0.203 |
| RR interval (ms) | 849.9 ± 118.8 | 874.2 ± 125.8 | 0.304 |
| RR variance (ms2) | 4806.2 ± 5381.2 | 5894.5 ± 6311.5 | 0.340 |
| RR LF a (ms2/Hz) | 1199.1 ± 1372.7 | 2423.9 ± 3635.9 | 0.166 |
| RR HF a (ms2/Hz) | 2365.3 ± 3824.9 | 2423.9 ± 3635.9 | 0.934 |
| RR LF nu (-) | 39.8 ± 20.4 | 42.0 ± 17.9 | 0.534 |
| RR HF nu (-) | 53.0 ± 21.1 | 48.6 ± 18.3 | 0.235 |
| RR LF/HF (-) | 1.27 ± 1.75 | 1.28 ± 1.52 | 0.976 |
| RR LF f (Hz) | 0.10 ± 0.03 | 0.10 ± 0.02 | 0.374 |
| RR HF f (Hz) | 0.30 ± 0.07 | 0.32 ± 0.06 | 0.113 |
| p_0v (-) | 15.6 ± 9.9 | 17.4 ± 12.6 | 0.409 |
| Resp HF f (Hz) | 0.31 ± 0.06 | 0.33 ± 0.06 | 0.139 |
| SAP Mean (mmHg) | 106.1 ± 12.3 | 113.6 ±11.1 | 0.001 |
| SAP total power (mmHg2) | 24.2 ± 18.9 | 32.1 ± 25.2 | 0.059 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Palombo, C.; Malacarne, M.; Pagani, M.; Federico, G.; Kozakova, M.; Zuccotti, G.; Lucini, D. Interaction between Autonomic Regulation, Adiposity Indexes and Metabolic Profile in Children and Adolescents with Overweight and Obesity. Children 2021, 8, 686. https://doi.org/10.3390/children8080686
Calcaterra V, Palombo C, Malacarne M, Pagani M, Federico G, Kozakova M, Zuccotti G, Lucini D. Interaction between Autonomic Regulation, Adiposity Indexes and Metabolic Profile in Children and Adolescents with Overweight and Obesity. Children. 2021; 8(8):686. https://doi.org/10.3390/children8080686
Chicago/Turabian StyleCalcaterra, Valeria, Carlo Palombo, Mara Malacarne, Massimo Pagani, Giovanni Federico, Michaela Kozakova, Gianvincenzo Zuccotti, and Daniela Lucini. 2021. "Interaction between Autonomic Regulation, Adiposity Indexes and Metabolic Profile in Children and Adolescents with Overweight and Obesity" Children 8, no. 8: 686. https://doi.org/10.3390/children8080686
APA StyleCalcaterra, V., Palombo, C., Malacarne, M., Pagani, M., Federico, G., Kozakova, M., Zuccotti, G., & Lucini, D. (2021). Interaction between Autonomic Regulation, Adiposity Indexes and Metabolic Profile in Children and Adolescents with Overweight and Obesity. Children, 8(8), 686. https://doi.org/10.3390/children8080686









