Transient Congenital Complete Heart Block: A Case Report
Abstract
:1. Introduction
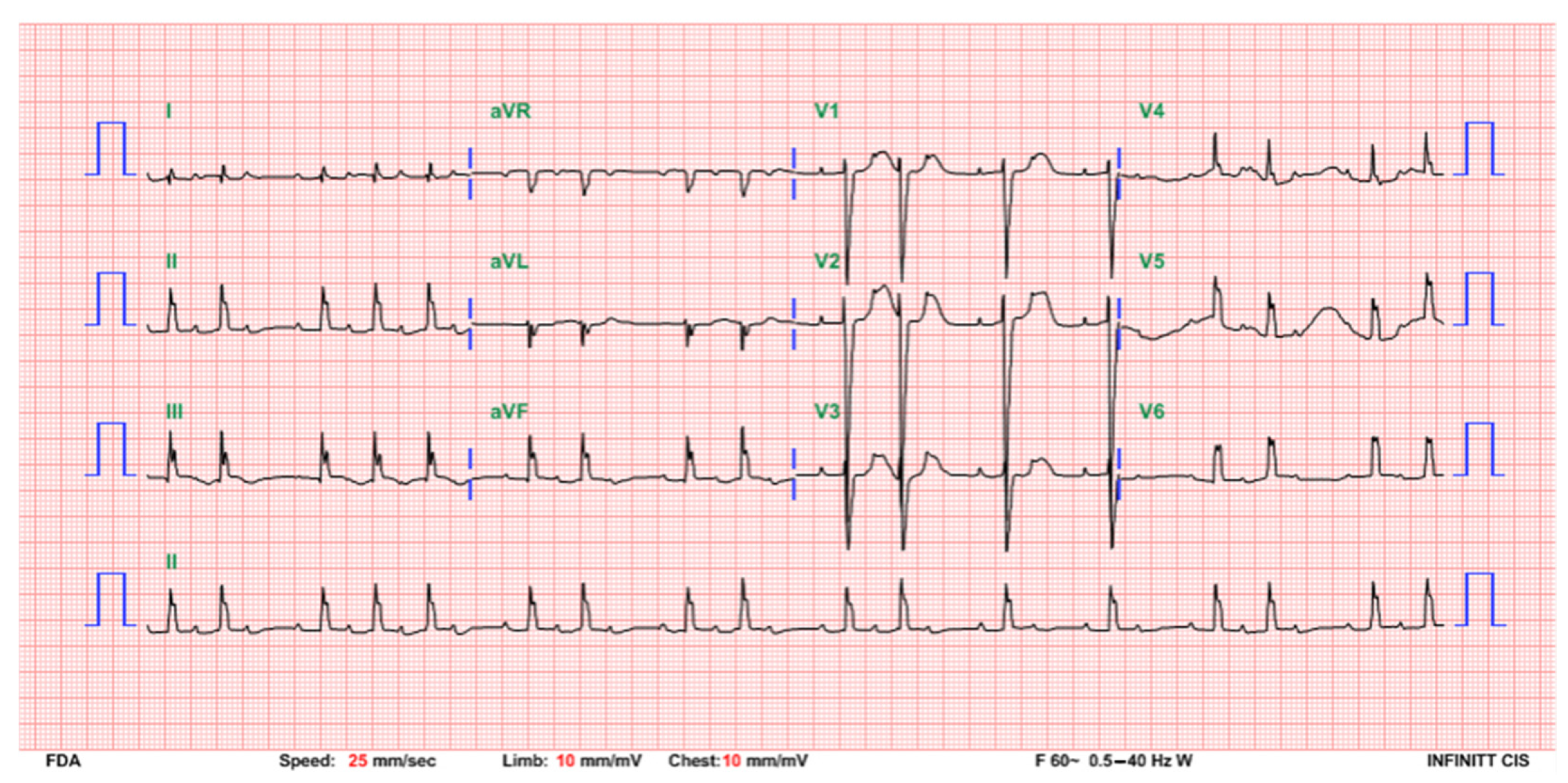
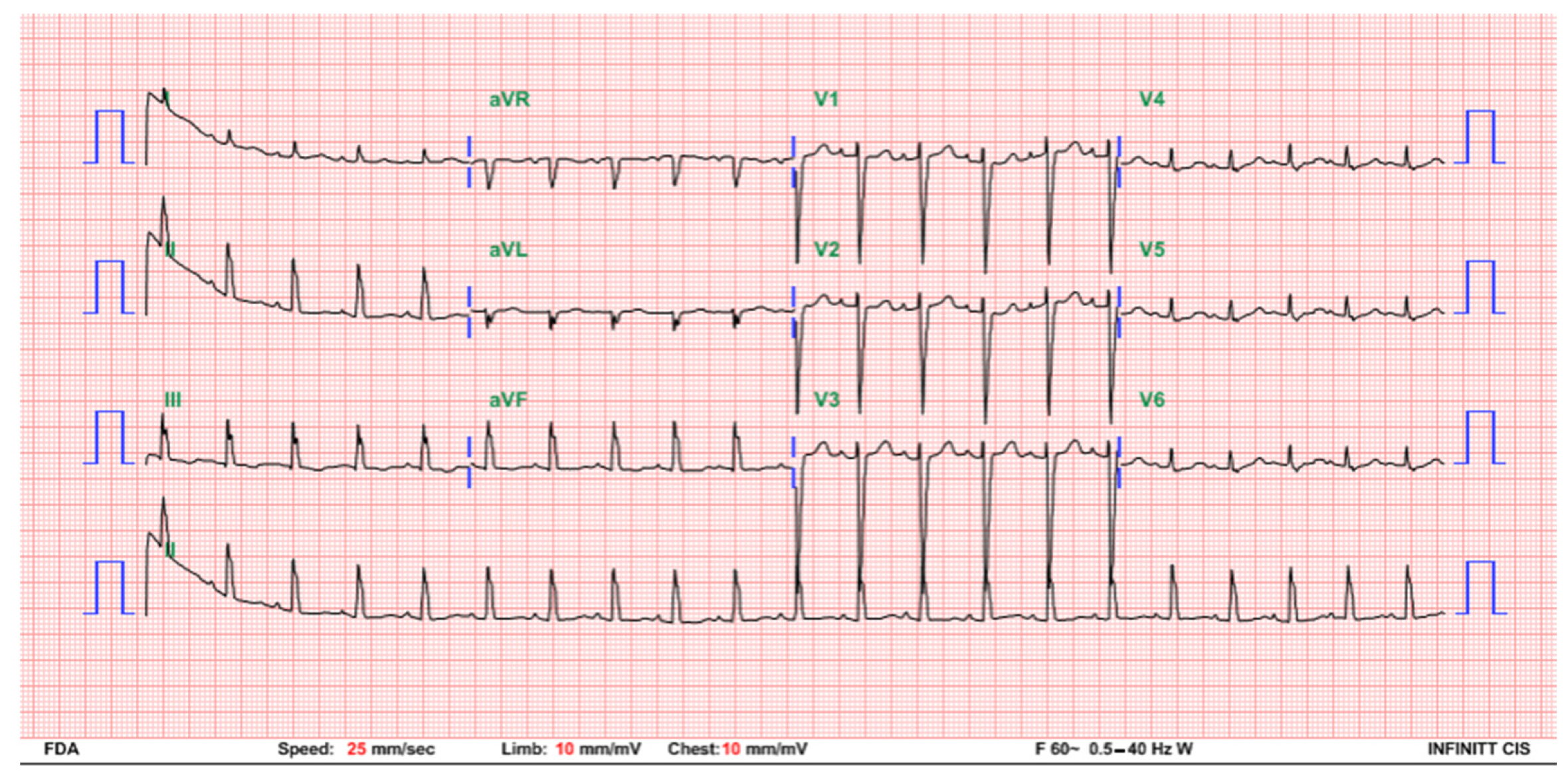
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baruteau, A.E.; Pass, R.H.; Thambo, J.B.; Behaghel, A.; Le Pennec, S.; Perdreau, E.; Combes, N.; Liberman, L.; McLeod, C.J. Congenital and childhood atrioventricular blocks: Pathophysiology and contemporary management. Eur. J. Pediatr. 2016, 175, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Jaeggi, E.; Ohman, A. Fetal and Neonatal Arrhythmias. Clin. Perinatol. 2016, 43, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.; Duncanson, L.; Glickstein, J.; Buyon, J. A review of congenital heart block. Images Paediatr. Cardiol. 2003, 5, 36–48. [Google Scholar] [PubMed]
- Martin, T.A. Congenital heart block: Current thoughts on management, morphologic spectrum, and role of intervention. Cardiol. Young 2014, 24 (Suppl. S2), 41–46. [Google Scholar] [CrossRef] [PubMed]
- Vanoni, F.; Lava, S.A.G.; Fossali, E.F.; Cavalli, R.; Simonetti, G.D.; Bianchetti, M.G.; Bozzini, M.A.; Agostoni, C.; Milani, G.P. Neonatal Systemic Lupus Erythematosus Syndrome: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2017, 53, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Buyon, J.P.; Hiebert, R.; Copel, J.; Craft, J.; Friedman, D.; Katholi, M.; Lee, L.A.; Provost, T.T.; Reichlin, M.; Rider, L.; et al. Autoimmune-associated congenital heart block: Demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J. Am. Coll. Cardiol. 1998, 31, 1658–1666. [Google Scholar] [CrossRef]
- De Caluwe, E.; Van de Bruaene, A.; Willems, R.; Troost, E.; Gewillig, M.; Rega, F.; Budts, W. Long-Term Follow-Up of Children with Heart Block Born from Mothers with Systemic Lupus Erythematosus: A Retrospective Study from the Database Pediatric and Congenital Heart Disease in University Hospitals Leuven. Pacing Clin. Electrophysiol. PACE 2016, 39, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Tunaoolu, F.S.; Karaaoac, A.T. Neonatal congenital heart block. Indian Pediatrics 2013, 50, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.; Gordon, P.; Rosenthal, E.; Simpson, J.; Miller, O.; Sharland, G. Isolated Complete Heart Block in the Fetus. Am. J. Cardiol. 2015, 116, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Epstein, A.E.; DiMarco, J.P.; Ellenbogen, K.A.; Estes, N.A., III; Freedman, R.A.; Gettes, L.S.; Gillinov, A.M.; Gregoratos, G.; Hammill, S.C.; Hayes, D.L.; et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2013, 61, e6–e75. [Google Scholar] [PubMed]
- Villain, E. Indications for pacing in patients with congenital heart disease. Pacing Clin. Electrophysiol. PACE 2008, 31 (Suppl. S1), S17–S20. [Google Scholar] [CrossRef] [PubMed]
- Jaeggi, E.T.; Hamilton, R.M.; Silverman, E.D.; Zamora, S.A.; Hornberger, L.K. Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block. A single institution’s experience of 30 years. J. Am. Coll. Cardiol. 2002, 39, 130–137. [Google Scholar] [CrossRef]
- Rahman, A.; Isenberg, D.A. Systemic lupus erythematosus. N. Engl. J. Med. 2008, 358, 929–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirén, M.K.; Julkunen, H.; Kaaja, R.; Kurki, P.; Koskimies, S. Role of HLA in congenital heart block: Susceptibility alleles in mothers. Lupus 1999, 8, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Escamilla, S.A.; Pettersen, M.D. Transient heart block in a neonate associated with previously undiagnosed maternal anti-Ro/SSA and anti-La/SSB antibodies. Pediatric Cardiol. 2007, 28, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Breur, J.M.; Oudijk, M.A.; Stoutenbeek, P.; Visser, G.H.; Meijboom, E.J. Transient non-autoimmune fetal heart block. Fetal Diagn. Ther. 2005, 20, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Delcò, C.; Beghetti, M.; Pfister, R.E. Vagal bradycardia at term. Acta Paediatr. 2009, 98, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Ballou, J.F.; Gray, B.P.; Mancuso, P. Bradycardia in a term newborn. J. Pediatr. Health Care 2014, 28, 456–460. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, Y.-T.; Wei, Y.-J.; Hsieh, M.-L.; Wang, J.-N.; Wu, J.-M. Transient Congenital Complete Heart Block: A Case Report. Children 2021, 8, 790. https://doi.org/10.3390/children8090790
Ju Y-T, Wei Y-J, Hsieh M-L, Wang J-N, Wu J-M. Transient Congenital Complete Heart Block: A Case Report. Children. 2021; 8(9):790. https://doi.org/10.3390/children8090790
Chicago/Turabian StyleJu, Ying-Tzu, Yu-Jen Wei, Ming-Ling Hsieh, Jieh-Neng Wang, and Jing-Ming Wu. 2021. "Transient Congenital Complete Heart Block: A Case Report" Children 8, no. 9: 790. https://doi.org/10.3390/children8090790





