Abstract
Telomere shortening can be enhanced upon human immunodeficiency virus (HIV) infection and by antiretroviral (ARV) exposures. The aim of this study was to evaluate the acute and long-term effect on telomere shortening of two ARV prophylaxes, lopinavir/ritonavir (LPV/r) and lamivudine (3TC), administered to children who are HIV-exposed uninfected (CHEU) to prevent HIV acquisition through breastfeeding during the first year of life, and to investigate the relationship between telomere shortening and health outcomes at six years of age. We included 198 CHEU and measured telomere length at seven days of life, at week-50 and at six years (year-6) using quantitative polymerase chain reaction. At week-50, telomere shortening was observed among 44.3% of CHEU, irrespective of the prophylactic treatment. Furthermore, this telomere shortening was neither associated with poor growth indicators nor neuropsychological outcomes at year-6, except for motor abilities (MABC test n = 127, β = −3.61, 95%CI: −7.08, −0.14; p = 0.04). Safety data on telomere shortening for infant HIV prophylaxis are scarce. Its association with reduced motor abilities deserves further attention among CHEU but also HIV-infected children receiving ARV treatment.
1. Introduction
Assaying the short- and long-term safety of antiretroviral (ARV) therapy is of utmost priority for public health guidelines on ARV regimens for HIV prevention and treatment. Given the urgent need to prevent and treat HIV, most ARV regimens have been established by a panel of experts using evidence from randomized, phase 3 clinical trials that have directly compared ARV regimens in research settings. Data on short-term safety of ARV therapy for pediatric use come from phase 1/2 safety and pharmacokinetic trials and nonrandomized, open-label studies [1]. Although pediatric ARV treatment regimens have proven effective in controlling HIV viral load and improving health outcomes in HIV-infected children, excess morbidities and mortalities are still reported in these pediatric populations [2,3,4]. The long-term side effects of ARV are poorly documented mainly due to the difficulty to truly discriminate drug effects from those of HIV exposure and/or infection.
Telomeres are the caretakers of our genetic information. These highly conserved DNA repeated sequences that cap the ends of the chromosomes prevent their degradation and their fusion, thus ensuring genomic stability [5]. Telomere shortening is a physiological event that occurs at each cell division in all somatic cells and is associated with age-related disorders [6]. Telomere dysfunction is the major cause of replicative senescence, but also initiates and maintains stem cell exhaustion and inflammation [7]. Many factors are associated with accelerated telomere shortening, such as male gender, smoking, alcohol consumption, ethnicity, obesity, sedentary lifestyle, genetic variants, socioeconomic status, as well as psychological stress [6,8]. Both ARV and HIV are known to promote telomere shortening. Chronic HIV-induced inflammation and free HIV viral particles themselves can enhance the production of reactive oxygen species damaging telomeric DNA and thus promoting telomere shortening [9,10,11]. Protease inhibitors (PI) [12,13,14,15,16], and particularly lopinavir [12], as well as nucleoside reverse transcriptase inhibitors induce reactive oxygen species production in several cell types or animal models [17,18,19,20]. A recent study reported a relationship between leucocyte telomere shortening and blood mitochondrial DNA content among HIV-infected or uninfected women, the greater the telomere shortening, the higher the mitochondrial DNA content [21].
If telomere shortening-associated premature aging was observed in adults living with HIV [22,23,24,25,26], relatively fewer studies investigated telomere length in younger populations. Of note, all but one of these studies were conducted outside Africa, the continent with the highest prevalence of HIV-infected children and children who are HIV-exposed uninfected (CHEU) [27]. One study reported shorter telomere length in early life for HIV-infected children when compared to children who are HIV-unexposed uninfected (CHUU), mostly of European origin [28], while another did not show a difference between HIV-infected and uninfected adolescents, mostly of black/African Canadian origin [29]. This discrepancy could possibly be explained by the age differences between cohorts and by the duration of ARV exposure. The impact of ARV exposure on telomere length was also addressed in CHEU in cross-sectional studies at different time points. Most of them described similar telomere length at birth between CHUU and CHEU who have been exposed in utero to zidovudine (AZT) [30], to the backbone AZT plus lamivudine (3TC) in combination with nevirapine (NVP), nelfinavir (NFV), or ritonavir-boosted protease inhibitor (PI) [31,32] or to other triple combination including abacavir (ABC), tenofovir disoproxyl fumarate (TDF) or emtricitabine (FTC) [31,32]. Later in childhood, at approximately two years of age, CHEU exposed in utero to AZT/3TC/NVP [28], AZT/3TC/PI [28,29], or TDF/FTC/PI [28] also presented similar telomere length when compared to CHUU. Some of these children also received short term (6-week) postnatal prophylaxis including AZT [28,29,30,32], AZT/3TC or AZT or ABC/FTC/NFV [29]. However, one study reported shorter telomere length in CHEU at six years of age that had been exposed in utero to 3TC plus lopinavir/ritonavir (LPV/r)-based regimens including ABC, AZT, or stavudine, compared to CHUU [33]. Only one longitudinal study showed telomere length shortening from birth to three years old among CHEU and CHUU [32]. More recently, ritonavir-boosted protease inhibitors were a risk factor for telomere shortening in HIV-infected pregnant women [34].
In a randomized controlled trial (PROMISE-PEP trial, NCT00640263), we tested the efficacy of single drug pre-exposure prophylaxis in infants to prevent mother-to-children transmission of HIV in breastfed CHEU [35,36]. The main outcomes of this study were percent HIV transmission and regimen safety. Furthermore, we conducted a follow-up study of these trial participants at six years of age to evaluate growth, clinical, and neurodevelopment outcomes post-ARV exposure (PROMISE-M&S trial, NCT03519503) [37]. These children received ARV prophylaxis of LPV/r or 3TC up to one year of age, providing a unique population to evaluate the impact of these drugs on telomere length among uninfected children, and without interference of other drugs from combined therapy.
The objectives of the present study were to compare the effects on telomere length of two ARV treatments, LPV/r and 3TC administered to prevent HIV acquisition in CHEU during the first year of life, and to assess whether telomere shortening at one year was predictive of impaired growth, clinical, and/or neurodevelopmental outcomes at six years of age.
2. Materials and Methods
2.1. Study Population
We performed a longitudinal observational study of CHEU enrolled in the PROMISE PEP trial (NCT00640263) and its follow-up study (PROMISE M&S, NCT03519503). The PROMISE PEP trial, conducted in Burkina Faso, South Africa, Uganda, and Zambia between November 2009 and May 2012, CHEU received daily LPV/r or 3TC prophylaxis to prevent mother-to-child-transmission of HIV-1 through breastfeeding [35,36]. These uninfected children at birth received seven days of NVP as per national guidelines and were thereafter randomly assigned to receive LPV/r or 3TC from seven days after birth (day-7) until one week after breastfeeding discontinuation, for a maximal duration of fifty weeks (W50). The PROMISE M&S study (recruitment from February 2017 to February 2018) consisted of a one- or two-day visit for a growth, clinical, and neuropsychological evaluation of the PROMISE PEP trial participants aged 5 to 7 years old who were HIV negative at the end of the prophylaxis period [37]. Among PROMISE M&S participants, we randomly selected 198 CHEU with 1:1 sex and prophylactic regimen ratios. The same selection criteria were used in a previous study investigating the mitochondrial DNA genotoxicity of the prophylaxis [38,39].
2.2. Sample Collection and DNA Extraction
Child dried blood spots (Whatman®903 cards, Lipomic Healthcare, New Delhi, India) were collected on study sites directly by heel prick for day-7 and week-50 time points (PROMISE PEP trial) or were processed from venous blood collected on EDTA tubes for the year-6 time point (PROMISE M&S trial). All dried blood spots were stored at −20 °C at the study sites in an individual zipped-pouch containing desiccant. DNA extraction was performed on 3-mm diameter punches (n = 3) using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. Extracted DNAs were stored at −80 °C.
2.3. Telomere Length Assay
2.3.1. Standard Curves and Quantitative Polymerase Chain Reaction
Telomere length measurement was performed by quantitative polymerase chain reaction, based on a method previously described by O’Callaghan and Fenech [40,41]. Two standard curves were used. The first curve was generated by a ten-fold serial dilution of a synthetic oligonucleotide of 14 repetitions of the telomeric sequence (TAAGGG) which determines the quantity of telomere in kilobases (kb) per reaction. The standard concentration ranged from 1.18 × 103 to 1.18 × 107 kb of telomere per reaction. The second curve consisted of a ten-fold serial dilution of a synthetic oligomer of the human single copy gene RPLP0 which estimates the number of copies of diploid genomes per reaction. The concentration ranged from 2.63 × 101 to 2.63 × 105 diploid genome copies. Oligomers and primers used for the quantitative polymerase chain reactions are described in the Supplementary materials (Supplementary Table S1).
Each quantitative polymerase chain reaction plate contained (i) both standard curves in duplicate, (ii) DNA (10 ng) from the Human Embryonic Kidney 293T cell line in triplicate to assess the intra- and inter-plate variations, and (iii) DNA (10 ng) extracted at day-7, week-50, and year-6 for each participant, in a single point assay for telomeric and RPLP0 regions. Amplification was performed on a LightCycler®480 II instrument (Roche, Basel, Switzerland) in a final volume of 30 µL containing 1× LightCycler®480 SYBR Green I Master (Roche, Basel, Switzerland) and 900 nM of both pairs of primers. The following thermal program was used: 95 °C for 10 min; 40 cycles of 95 °C for 15 s, 60 °C for 30 s, 72 °C for 30 s, and ended by a melting curve.
2.3.2. Telomere Data Analysis
We performed absolute quantification analysis using the Fit Points method on a LightCycler®480 software (Roche, Basel, Switzerland). The threshold line was set at the beginning of the exponential phase of amplification. Telomere length per cell was calculated by dividing the Kb/reaction for telomere by the RPLP0 diploid copies/reaction. Results were expressed in Kb telomere per cell.
qPCR efficiency, intra- and inter-plate variation coefficients, quality control of quantitative polymerase chain reactions, and outlier identification are described in the Supplementary materials.
2.4. Statistical Analysis
We described the participant characteristics using either means with standard deviation (SD) or medians with interquartile range ([IQR]) for continuous variables, and percentages for categorical variables.
Our primary objective was to investigate the short-term and long-term effects of ARV prophylaxis on telomere length. We first described the telomere length at day-7, i.e., before the initiation of prophylactic regimens, and compared this parameter by study site and gender using the Kruskal–Wallis test applying the Dwass-Steel-Critchlow-Fligner method for all pairwise comparisons, and a Wilcoxon Mann–Whitney test, respectively.
Second, we addressed variation of telomere length between t0 (day-7 or week-50) and t1 (week-50 or year-6). We compared telomere length between the two time points using a Wilcoxon signed-rank test, and defined telomere shortening as follows:
Telomere shortening = [(TL at t1) + 4.03% × (TL at t1)] − [(TL at t0) − 4.03% × (TL at t0)] < 0, where 4.03% was the estimated experimental error (see Supplementary Materials). This telomere shortening threshold closely approaches the physiological decline in telomere length reported by Ajaykumar et al. among Canadian CHEU [32].
We compared the proportion of CHEU with telomere shortening at week-50 (t0 = day-7) or at year-6 (t0 = week-50) between the two prophylactic regimens using a Chi-square test. Analyses for telomere shortening between week-50 and year-6 were restricted to three out of the four study sites (n = 128). The three sites were Burkina Faso, Uganda, and Zambia, because samples for South Africa were not collected at year-6.
Throughout the analyses, we compared the two prophylactic regimens, LPV/r and 3TC, using Student’s t-test or Wilcoxon Mann–Whitney test for continuous variables, and Chi-square test or Fisher’s exact test for categorical variables.
Our secondary objective was to assess whether telomere shortening at week-50 was associated with child health impairments at year-6 and with higher mitochondrial DNA content. For this purpose, we first investigated the association between telomere shortening at week-50 and growth indicators (weight-for-age Z-score, height-for-age Z-score and body mass index Z-score) as well as neuropsychological performances (global scores obtained from the Strengths and Difficulties Questionnaire, SDQ-25; the Test Of Variable of Attention, TOVA; the Movement Assessment Battery for Children second edition, MABC-2 and the Kaufman Assessment Battery for Children second edition, KABC-II) at year-6, using linear regressions. Methodology for scores analysis and main findings is described elsewhere [37]. A square transformation was applied to SDQ-25 score in order to obtain a normal distribution. We also investigated the association between hospital admissions since week-50 and telomere shortening at week-50 using Poisson regressions with robust error variance. Growth analyses were adjusted for the prophylaxis regimen, the age of the child, the gender, the gestational age, the study site, the type of income generating activities, the mother’s educational level, and the number of children under five years old living in the household. Neuropsychological and hospital admission analyses were adjusted for the same confounders plus the child’s weight and height at year-6. All of these analyses were restricted to Burkina Faso, Uganda, and Zambia for the above mentioned reason.
Second, we investigated the association between telomere length and shortening with mitochondrial DNA content using linear regression in an analysis restricted to CHEU from Burkina Faso and Uganda (n = 73). Zambia was excluded from the analyses because we previously reported an interaction between mitochondrial DNA content and platelet count [38,39]. Mitochondrial DNA depletion was defined as a 50% or more decrease in mitochondrial DNA copy number per cell from day-7 to week-50 [38]. Mitochondrial DNA content at day-7, week-50, and year-6 were incremented per 50 copies. Analyses at week-50 and year-6 were adjusted for the type of prophylactic regimen. To address whether telomere shortening at week-50 was associated with mitochondrial DNA depletion at week-50, we used log-binomial regressions adjusted for the type of prophylactic regimen.
Statistical analyses were performed using SAS studio (Copyright © 2021–2016, SAS Institute Inc., Cary, NC, USA). The forest plot was drawn using GraphPad software v7.0 (Copyright © 2021–2018).
3. Results
3.1. Characteristics of the Study Population
One hundred and sixty-seven CHEU were enrolled in our study after quality control of DNA (Supplementary Figure S1). The characteristics of analyzed CHEU did not differ from those who were not selected, with the exception of lower platelet count (Supplementary Table S2). Children were equally distributed between the four sites of the trial (Table 1). Most of them were born at term. At day-7, mean height and weight were 49.6 ± 2.0 and 3.3 ± 0.5, respectively. However, 10.6% to 15.4% of CHEU had either stunted growth, were wasted, or underweight. Hemoglobin concentration and blood cell concentrations were within the normal range for more than 92.0% of CHEU. Although no difference between prophylactic groups was observed, the prevalence of CHEU with stunting or wasting was roughly two-fold higher for those who received LPV/r as compared to those who received 3TC. Characteristics of CHEU according to study sites are described in Supplementary Tables S3–S6.

Table 1.
Children’s characteristics at randomization (day-7).
According to the national prevention of mother-to-child transmission guidelines prevailing at the time the trial was conducted (2009–2012), none of the mothers had received an ARV treatment before the first antenatal visit. As per inclusion criteria, CD4 cell count was above 350 cells/mm3 (Table 2) and no mother was on ARV therapy. Zidovudine (ZDV) prophylaxis was the main ARV regimen given during pregnancy, followed by a combination ZDV plus 3TC. Viral load was controlled (less than 1000 copies/mL) in 59.3% of the mothers at day-7. Approximately 80.0% of mothers had an income-generating activity and 85.0% had attended school. Almost all mothers did not smoke during pregnancy or during the breastfeeding period (97.8 and 98.6%, respectively). However, more than one-third reported alcohol consumption during pregnancy. No maternal differences between prophylactic groups was observed at randomization. However, there seemed to be fewer mothers who had attended school seemed in the 3TC group as compared to those in the LPV/r group, and inversely for those who had a regular income-generating activity. Characteristics of the mothers according to study sites are described in Supplementary Tables S7–S10.

Table 2.
Maternal characteristics at randomization (day-7) and during the PROMISE PEP trial follow-up.
3.2. Telomere Length at Day-7
At randomization (day-7), median telomere length was 294 kb/cell with an interquartile range of 144 to 438 kb/cell (Table 3). Median telomere lengths (in kb/cell) for the 3TC and for the LPV/r groups were 270 [137; 434] and 321 [152; 445], respectively (p = 0.29). Telomere length was significantly different between sites (p < 0.01), CHEU from South Africa having the lowest telomere length compared to CHEU from the three other sites which were similar (p < 0.01 for South Africa versus Burkina Faso, South Africa versus Uganda and South Africa versus Zambia; p = 0.51 for Burkina Faso versus Uganda; p = 1.00 for Burkina Faso versus Zambia; p = 0.58 for Uganda versus Zambia). No statistical difference was observed in telomere length according to the 3TC and for the LPV/r groups at each site. No difference according to gender was observed (Supplementary Table S11).

Table 3.
Children’ telomere length at day-7.
3.3. Telomere Length after One Year of Prophylaxis
Characteristics of CHEU at week-50 are described in Supplementary Table S12. Overall, after one year of prophylaxis, telomere length remained stable as compared to baseline (day-7) values with a median of 294 kb/cell [141; 417] (p = 0.62) (Table 4). However, when dichotomizing telomere length by profile, 74 CHEU (44.3%) had telomere shortening at week-50, 34 (45.3%) were in the LPV/r group, and 40 (43.5%) were in the 3TC group (p = 0.81).

Table 4.
Telomere length at week-50 and proportion of CHEU with telomere shortening at week-50.
3.4. Telomere Length at Six Years Old
Characteristics of CHEU with available samples at year-6 (n = 128 CHEU from Burkina Faso, Uganda and Zambia) are described in Supplementary Table S13. No difference between prophylactic groups was observed. Among these children, telomere length decreased from week-50, for which the median was observed at 333 kb/cell [252; 444], to reach a median of 274 kb/cell [182; 368] at year-6 (p = 0.58). Eighty-six CHEU (67.2%) had telomere shortening at year-6, 43 in each prophylactic group (p = 0.61). Among these children, 25 (29.1%) had already demonstrated telomere shortening during the first year of life.
3.5. Health Outcomes at Six Years Old among CHEU with Telomere Shortening at Week-50
Characteristics at week-50 of CHEU with or without telomere shortening are described in Supplementary Table S13. No difference between the two populations was observed except for the gestational age (p < 0.01). Furthermore, the prevalence of prematurity seemed to be two-fold higher among CHEU without telomere shortening (p = 0.20) as compared to those with telomere shortening.
Linear and Poisson regression models with robust error variance after adjustment for confounders showed that telomere shortening at week-50 was neither associated with poor growth indicators at year-6 (Figure 1), nor hospital admissions since week-50 (PR = 0.75, 95%CI: 0.42, 1.35; p = 0.34). However, among neuropsychological performances, motor capacities assessed by the MABC-2 test were negatively associated with telomere shortening at week-50 (β = −3.61, 95%CI: −7.08, −0.14); p = 0.04) (Figure 1).
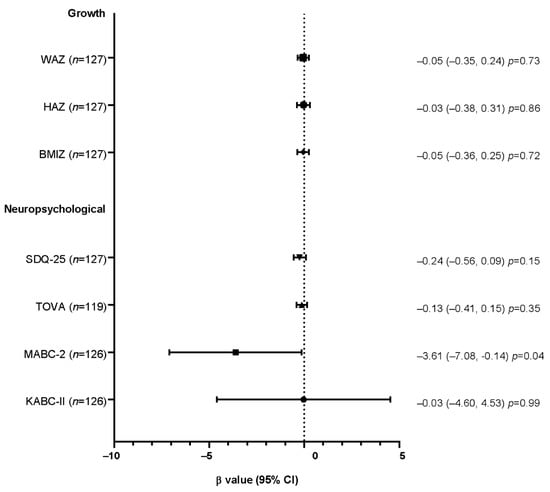
Figure 1.
Forest plot of the association between growth and neuropsychological outcomes at year-6 with telomere shortening at week-50, assessed by linear regressions.
β values and their confidence intervals for the final global scores for the SDQ-25, TOVA, MABC-2, and KABC-II neuropsychological tests and are presented in Figure 1. A square transformation was applied to the SDQ-25 score. Growth analyses were adjusted for the prophylactic regimen, the age of the child, the gender, the study site, the type of income generating activities, the mother’s education level, and the number of children under five years old living in the household. Neuropsychological analyses were adjusted for the same cofounders plus the child’s height and weight at year-6. Abbreviations: WAZ, weight-for-age Z-score; HAZ, height-for-age Z-score; BMIZ, body mass index Z-score; SDQ-25, Strength and Difficulties Questionnaire; TOVA, Test of Variable Of Attention; MABC-2, Movement Assessment Battery for Children second edition; KABC-II, Kaufman Assessment Battery for Children second edition; CI, confidence interval.
3.6. Relationship between Telomere Length and Shortening with Mitochondrial DNA Content
We investigated the relationship between telomere length and mitochondrial DNA content in an analysis restricted to CHEU from Burkina Faso and Uganda (n = 73). At day-7, linear regression models showed that telomere length was not associated with mitochondrial DNA content (n = 73, β1 = 1.52, 95%CI: −9.28, 12.33; p = 0.78). At week-50 and after adjustment for the prophylactic regimen, we observed that the lower the mitochondrial DNA content, the higher the telomere length (n = 73, β1 = −7.2, 95%CI: −13.8, −0.72; p = 0.03), but at year-6 there was no significant association (n = 73, β1 = 2.95, 95%CI: −8.89, 14.78; p = 0.63). Finally, telomere shortening at week-50 was not associated with mitochondrial DNA depletion at week-50 after adjustment for the prophylactic regimen (n = 73, PR = 1.37, 95%CI: 0.86, 2.19; p = 0.19).
4. Discussion
In this study, CHEU receiving 3TC or LPV/r prophylaxis during breastfeeding exhibited similar telomere dynamics at one year of age. The prevalence of CHEU with telomere shortening at week-50 was similar between the two prophylactic regimens. Telomere length did not significantly decrease until the six-year benchmark. Finally, those identified with telomere shortening at week-50 did not demonstrate impaired growth or poorer clinical outcomes at year-6, but were associated with motor impairment.
Given the absence of a control group not receiving any prophylactic treatment in this study, these observations suggest that both of the study drugs could have the same effect on telomere length, or that the telomere attrition we report is physiological. Previous reports have failed to demonstrate ARV toxicity on telomere length among CHEU exposed in utero or for a short period post-partum (28–33), which could suggest that one year of 3TC or LPV/r prophylaxis does not modify the rate of telomere shortening. We also tentatively compared these observations with those that have already been published. It is noteworthy that there is an absence of consensus regarding a reference value for telomere length among different populations as well as within specific populations. Similarly, a consensus for the definition of telomere shortening is lacking. However, it is worth mentioning that there exists reassuring data in the use of short course, i.e., six weeks, infant ZDV prophylaxis on telomere length. Gianesin et al. showed similar telomere length between CHEU receiving prophylaxis compared to CHUU [28]. Furthermore, one longitudinal study did not show variation in telomere length from birth to 31 days among 58 Canadian CHEU receiving ZDV [32]. However, the latter study reported a quicker telomere length decrease during the first 40 weeks of life among 214 CHEU compared to CHUU [32]. Altogether, ARVs used in prophylaxis regimens are unlikely associated with telomere shortening.
We also took advantage of the cohort design of our study to evaluate the impact of telomere shortening early in life on child health and neurodevelopment. The definition of early biomarkers characterizing children with poor health outcomes is a major public health concern for CHEU [42]. A negative association between telomere shortening and motor capacity was observed. Reasons for such an association remain obscure but we believed it is worth repeating in different cohorts in order to more definitively conclude on its prognostic value.
This work is unique as it described telomere length evolution among an African cohort of CHEU with little interference with other maternal ARV exposure. Furthermore, given that the child prophylaxis consisted of a single drug, we were able to evaluate each drug individually and not in combination with other molecules. To date, only one other study has investigated telomere length among South African CHEU, using a cross-sectional design [33]. In addition, we followed CHEU to a later age than a previously published longitudinal study describing telomere length, which observed children until three years of age [32], and conducted original investigations on the relationship between telomere shortening and growth and neuropsychological development at school age. Furthermore, given that a small proportion of mothers has smoked during pregnancy and breastfeeding, our analysis obviates the major confounding factor which was commonly encountered in other studies among CHEU. Moreover, we benefited from the randomization of the PROMISE PEP trial thus minimizing the risk of selection bias, participant bias, and confounding factors bias. Telomere length measurement was well-performed according to a robust, standardized method with similar or lower inter- and intra-assay coefficients of variation as compared with the other studies reported so far among CHEU [27,29,31,32].
However, our study also presents limitations. First, in contrast to other studies carried out among CHEU or HIV-infected children that have assessed telomere length from whole venous blood, we measured telomere length from dried blood spots. A few studies support dried blood spots as a less invasive method for measuring this parameter but they report a high correlation between relative telomere length in dried bloods spots and whole venous blood [43,44]. Second, we have not been able to observe an effect of the gender on telomere length. Yet, the male gender is a well-documented factor associated with shorter telomere length in comparison to females in the general population [45,46,47]. However, it remains unclear whether this gender effect is already present at birth. Some studies have reported this observation in newborns [48,49] while others have not been able to find such a difference between male and female babies [50,51]. Among CHEU, one study reports an association between male gender and shorter telomere length at birth [32], which could not have been related to a sample size effect (n = 106 versus n = 167). However, one could speculate that this gender effect could be related to the ethnic disparity between the two studies, the first one encompassing CHEU from different origins (Indigenous, Black/African Canadian, White and Asian) while we enrolled only African CHEU. Ethnicity is a known factor associated with telomere length. Particularly, populations of African descent appear to have longer telomeres than Caucasian populations [52,53,54,55]. One study described similar telomere length between healthy black females and male newborns, as we observed in this study [54]. Larger studies are clearly warranted to further investigate both gender and ethnic differences concerning telomere length in newborns and children. Another limitation of our study is that, interestingly, telomere length at day-7 was around two-thirds lower for CHEU from South Africa as compared to those from the three other sites. To date, no study has reported such a difference due to the fact that none have been multi-centric. A recent study evaluated telomere length among seven Sub-Saharan populations (two ancestries from Botswana and Tanzania and three ancestries from Ethiopia) and showed that the San ancestry population from Botswana had a greater telomere length when compared to the six other populations. Third, we were unable to assess with accuracy at what point in time, after the first year of life, telomere shortening occurred as we did not have intermediate time points between the first and sixth year of life. Fourth, some information was self-reported, including previous clinical consultations and hospital admissions, smoking during pregnancy and breastfeeding, alcohol consumption during pregnancy, type of income, and the mother’s education. We also lack information on several factors which are known to be associated with telomere shortening such as paternal age [56,57,58], substance abuse [59,60,61], and cytomegalovirus infection [62]. Finally, we could not perform long-term follow-up of CHEU from South Africa because samples were not collected and stored.
In conclusion, our study revealed no significant difference regarding telomere length between the two prophylactic treatments, 3TC and LPV/r, after one year of treatment. However, the association between telomere shortening at W50 and motor impairment at year-6 deserves further attention for CHEU as well as for HIV-infected children.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/children8090796/s1, Figure S1. Analysis flow for telomere length measurement. Table S1. Oligomers and primers used for telomere length measurement by quantitative polymerase chain reaction. Table S2. Characteristics of CHEU analyzed in the study versus CHEU not selected, at randomization (day-7). Table S3. Children’s characteristics at randomization (day-7) for Burkina Faso. Table S4. Children’s characteristics at randomization (day-7) for South Africa. Table S5. Children’s characteristics at randomization (day-7) for Uganda. Table S6. Children’s characteristics at randomization (day-7) for Zambia. Table S7. Maternal characteristics at randomization (day-7) and during follow-up for the PROMISE PEP trial in Burkina Faso. Table S8. Maternal characteristics at randomization (day-7) and during follow-up for the PROMISE PEP trial in South Africa. Table S9. Maternal characteristics at randomization (day-7) and during follow-up for the PROMISE PEP trial in Uganda. Table S10. Maternal characteristics at randomization (day-7) and during follow-up for the PROMISE PEP trial in Zambia. Table S11. Children’ telomere length at day-7 according to gender. Table S12. Children’s characteristics at week-50. Table S13. Characteristics of enrolled CHEU aged six years of age from the PROMISE M&S trial. Table S14. Characteristics at week-50 of CHEU with or without telomere shortening at week-50.
Author Contributions
Conceptualization, N.N., T.T., P.V.d.P. and J.-P.M.; methodology, S.E.-D. and N.N.; validation S.E.-D. and J.-P.M.; formal analysis, A.M. and S.E.-D.; investigation, A.M., A.V., N.M., M.S.-M., G.N., J.K.T., C.K. and A.G.; data curation, A.M. and S.E.-D.; writing original draft preparation, A.M., A.V., N.N. and J.-P.M.; writing, review and editing, A.M., A.V., N.N., S.E.-D., N.M., G.N., J.K.T., A.G., T.T., P.V.d.P. and J.-P.M.; supervision, N.M., M.S.-M., G.N., J.K.T., C.K. and A.G.; funding acquisition, N.N., P.V.d.P., T.T. and J.-P.M. All authors have read and agreed to the published version of the manuscript.
Funding
The PROMISE-PEP trial was supported by the French National Agency for Research on AIDS and Viral Hepatitis (ANRS#12274), the European and Developing Countries Clinical Trials Partnership (#CT.2006.33020.004), and the Research Council of Norway (GlobVac grant #183600). The PROMISE M&S trial was funded by the Pierre Bergé endowment fund in collaboration with SIDACTION, grant number AP-FPB-2013-2/09, and sponsored by the French National Agency for Research on AIDS and Viral Hepatitis (ANRS#12174 and ANRS#12341). A.M. is the beneficiary of a doctoral scholarship from the French National Agency for Research on AIDS and Viral Hepatitis (ANRS#12174-B90).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Biomedical Research Ethics Committee in Zambia (N° 008-02-080 (15 January 2011) & 002-12-15 (24 June 2016)), the Uganda National Council for Science and Technology (N° HS 470 (31 July 2009) & HS1988 (29 February 2016)), the Stellenbosh University ethics committees for South Africa (N° M09/11/043 (14 June 2010)) and the Ethical Committee for Health Research in Burkina Faso (N° 2008-039 (29 January 2008) & 2016-4-041 (14 April 2016)).
Informed Consent Statement
Written informed consents were obtained from the mother or the legal representative prior to enrolment in the ANRS 12174 PROMISE-PEP trial (NCT00640263) and the PROMISE M&S trial (NCT03519503). The consent included the use of the banked samples for ancillary studies related to HIV infection which were approved by the scientific advisory board.
Data Availability Statement
The full data set presented in this study is available on request from the corresponding author.
Acknowledgments
We would like to thank all the participating mothers, children, and caregivers, and all the members of the PROMISE Consortium.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. Available online: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/PediatricGuidelines.pdf (accessed on 26 August 2021).
- Ahmed, I.; Lemma, S. Mortality among Pediatric Patients on HIV Treatment in Sub-Saharan African Countries: A Systematic Review and Meta-Analysis. BMC Public Health 2019, 19, 149. [Google Scholar] [CrossRef] [Green Version]
- Slogrove, A.L.; Goetghebuer, T.; Cotton, M.F.; Singer, J.; Bettinger, J.A. Pattern of Infectious Morbidity in HIV-Exposed Uninfected Infants and Children. Front. Immunol. 2016, 7, 164. [Google Scholar] [CrossRef]
- Brennan, A.T.; Bonawitz, R.; Gill, C.J.; Thea, D.M.; Kleinman, M.; Useem, J.; Garrison, L.; Ceccarelli, R.; Udokwu, C.; Long, L.; et al. A Meta-Analysis Assessing All-Cause Mortality in HIV-Exposed Uninfected Compared with HIV-Unexposed Uninfected Infants and Children. AIDS 2016, 30, 2351–2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and Telomere Length: A General Overview. Cancers 2020, 12, 558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, K.; Vasu, V.; Griffin, D. Telomere Biology and Human Phenotype. Cells 2019, 8, 73. [Google Scholar] [CrossRef] [Green Version]
- Chakravarti, D.; LaBella, K.A.; DePinho, R.A. Telomeres: History, health, and hallmarks of aging. Cell 2021, 184, 306–322. [Google Scholar] [CrossRef]
- Martens, D.S.; Janssen, B.G.; Bijnens, E.M.; Clemente, D.B.P.; Vineis, P.; Plusquin, M.; Nawrot, T.S. Association of Parental Socioeconomic Status and Newborn Telomere Length. JAMA Netw. Open 2020, 3, e204057. [Google Scholar] [CrossRef] [PubMed]
- Bellon, M.; Nicot, C. Telomere Dynamics in Immune Senescence and Exhaustion Triggered by Chronic Viral Infection. Viruses 2017, 9, 289. [Google Scholar] [CrossRef] [Green Version]
- Pace, G.W.; Leaf, C.D. The Role of Oxidative Stress in HIV Disease. Free Radic. Biol. Med. 1995, 19, 523–528. [Google Scholar] [CrossRef]
- Deeks, S.G.; Triant, V.A.; Lee, H.; Hadigan, C.; Grinspoon, S.K.; Schaftenaar, F.; Frodermann, V.; Kuiper, J.; Lutgens, E.; Simmons, R.P.; et al. HIV Infection, Inflammation, Immunosenescence, and Aging. AIDS 2013, 27, 1263–1272. [Google Scholar] [CrossRef] [Green Version]
- Taura, M.; Kariya, R.; Kudo, E.; Goto, H.; Iwawaki, T.; Amano, M.; Suico, M.A.; Kai, H.; Mitsuya, H.; Okada, S. Comparative Analysis of ER Stress Response into HIV Protease Inhibitors: Lopinavir but Not Darunavir Induces Potent ER Stress Response via ROS/JNK Pathway. Free Radic. Biol. Med. 2013, 65, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Gratton, R.; Tricarico, P.M.; Guimaraes, R.L.; Celsi, F.; Crovella, S. Lopinavir/Ritonavir Treatment Induces Oxidative Stress and Caspaseindependent Apoptosis in Human Glioblastoma U-87 MG Cell Line. Curr. HIV Res. 2018, 16, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Du, L.; Pham, P.; Zhu, B.; Jiang, S. Nelfinavir, an HIV Protease Inhibitor, Induces Apoptosis and Cell Cycle Arrest in Human Cervical Cancer Cells via the ROS-Dependent Mitochondrial Pathway. Cancer Lett. 2015, 364, 79–88. [Google Scholar] [CrossRef]
- Gibellini, L.; De Biasi, S.; Pinti, M.; Nasi, M.; Riccio, M.; Carnevale, G.; Cavallini, G.M.; Sala De Oyanguren, F.J.; O’Connor, J.E.; Mussini, C.; et al. The Protease Inhibitor Atazanavir Triggers Autophagy and Mitophagy in Human Preadipocytes. AIDS 2012, 26, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Ganta, K.K.; Chaubey, B. Endoplasmic Reticulum Stress Leads to Mitochondria-Mediated Apoptosis in Cells Treated with Anti-HIV Protease Inhibitor Ritonavir. Cell Biol. Toxicol. 2019, 35, 189–204. [Google Scholar] [CrossRef]
- Blas-García, A.; Martí-Rodrigo, A.; Víctor, V.M.; Polo, M.; Alegre, F.; Funes, H.A.; Apostolova, N.; Esplugues, J.V. The Purine Analogues Abacavir and Didanosine Increase Acetaminophen-Induced Hepatotoxicity by Enhancing Mitochondrial Dysfunction. J. Antimicrob. Chemother. 2016, 71, 916–926. [Google Scholar] [CrossRef] [Green Version]
- Velsor, L.W.; Kovacevic, M.; Goldstein, M.; Leitner, H.M.; Lewis, W.; Day, B.J. Mitochondrial Oxidative Stress in Human Hepatoma Cells Exposed to Stavudine. Toxicol. Appl. Pharmacol. 2004, 199, 10–19. [Google Scholar] [CrossRef]
- Day, B.J.; Lewis, W. Oxidative Stress in NRTI-Induced Toxicity: Evidence from Clinical Experience and Experiments in Vitro and in Vivo. Cardiovasc. Toxicol. 2004, 4, 207–216. [Google Scholar] [CrossRef]
- Smith, R.L.; Tan, J.M.E.; Jonker, M.J.; Jongejan, A.; Buissink, T.; Veldhuijzen, S.; Van Kampen, A.H.C.; Brul, S.; Van Der Spek, H. Beyond the Polymerase-γ Theory: Production of ROS as a Mode of NRTI-Induced Mitochondrial Toxicity. PLoS ONE 2017, 12, e0187424. [Google Scholar] [CrossRef]
- Hsieh, A.Y.Y.; Kimmel, E.; Pick, N.; Sauvé, L.; Brophy, J.; Kakkar, F.; Bitnun, A.; Murray, M.C.M.; Côté, H.C.F. Inverse Relationship between Leukocyte Telomere Length Attrition and Blood Mitochondrial DNA Content Loss over Time. Aging 2020, 12, 15196–15221. [Google Scholar] [CrossRef]
- Nichols, W.S.; Schneider, S.; Chan, R.C.K.; Farthing, C.F.; Daar, E.S. Increased CD4+ T-Lymphocyte Senescence Fraction in Advanced Human Immunodeficiency Virus Type 1 Infection. Scand. J. Immunol. 1999, 49, 302–306. [Google Scholar] [CrossRef] [Green Version]
- Minami, R.; Takahama, S.; Yamamoto, M. Correlates of Telomere Length Shortening in Peripheral Leukocytes of HIV-Infected Individuals and Association with Leukoaraiosis. PLoS ONE 2019, 14, e0218996. [Google Scholar] [CrossRef]
- Babu, H.; Ambikan, A.T.; Gabriel, E.E.; Akusjärvi, S.S.; Palaniappan, A.N.; Sundaraj, V.; Mupanni, N.R.; Sperk, M.; Cheedarla, N.; Sridhar, R.; et al. Systemic Inflammation and the Increased Risk of Inflamm-Aging and Age-Associated Diseases in People Living with HIV on Long Term Suppressive Antiretroviral Therapy. Front. Immunol. 2019, 10, 1965. [Google Scholar] [CrossRef] [Green Version]
- Pathai, S.; Lawn, S.D.; Gilbert, C.E.; McGuinness, D.; McGlynn, L.; Weiss, H.A.; Port, J.; Christ, T.; Barclay, K.; Wood, R.; et al. Accelerated Biological Ageing in HIV-Infected Individuals in South Africa: A Case-Control Study. AIDS 2013, 27, 2375–2384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.C.Y.; Leung, J.M.; Ngan, D.A.; Nashta, N.F.; Guillemi, S.; Harris, M.; Lima, V.D.; Um, S.J.; Li, Y.; Tam, S.; et al. Absolute Leukocyte Telomere Length in HIV-Infected and Uninfected Individuals: Evidence of Accelerated Cell Senescence in HIV-Associated Chronic Obstructive Pulmonary Disease. PLoS ONE 2015, 10, e0124426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UNAIDS AIDSinfo. Available online: https://aidsinfo.unaids.org/ (accessed on 24 November 2020).
- Gianesin, K.; Noguera-Julian, A.; Zanchetta, M.; Del Bianco, P.; Petrara, M.R.; Freguja, R.; Rampon, O.; Fortuny, C.; Camós, M.; Mozzo, E.; et al. Premature Aging and Immune Senescence in HIV-Infected Children. AIDS 2016, 30, 1363–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Côté, H.C.F.; Soudeyns, H.; Thorne, A.; Alimenti, A.; Lamarre, V.; Maan, E.J.; Sattha, B.; Singer, J.; Lapointe, N.; Money, D.M.; et al. Leukocyte Telomere Length in HIV-Infected and HIV-Exposed Uninfected Children: Shorter Telomeres with Uncontrolled HIV Viremia. PLoS ONE 2012, 7, e39266. [Google Scholar] [CrossRef]
- Poirier, M.C.; Divi, R.L.; Al-Harthi, L.; Olivero, O.A.; Nguyen, V.; Walker, B.; Landay, A.L.; Walker, V.E.; Charurat, M.; Blattner, W.A. Long-Term Mitochondrial Toxicity in HIV-Uninfected Infants Born to HIV-Infected Mothers. JAIDS J. Acquir. Immune Defic. Syndr. 2003, 33, 175–183. [Google Scholar] [CrossRef]
- Imam, T.; Jitratkosol, M.H.J.; Soudeyns, H.; Sattha, B.; Gadawski, I.; Maan, E.; Forbes, J.C.; Alimenti, A.; Lapointe, N.; Lamarre, V.; et al. Leukocyte Telomere Length in HIV-Infected Pregnant Women Treated with Antiretroviral Drugs during Pregnancy and Their Uninfected Infants. JAIDS J. Acquir. Immune Defic. Syndr. 2012, 60, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Ajaykumar, A.; Soudeyns, H.; Kakkar, F.; Brophy, J.; Bitnun, A.; Alimenti, A.; Albert, A.Y.K.; Money, D.M.; Côté, H.C.F.; CIHR Team in Cellular Aging and HIV Comorbidities in Women and Children. Leukocyte Telomere Length at Birth and during the Early Life of Children Exposed to but Uninfected with HIV after In Utero Exposure to Antiretrovirals. J. Infect. Dis. 2018, 217, 710–720. [Google Scholar] [CrossRef]
- Shiau, S.; Strehlau, R.; Shen, J.; Violari, A.; Patel, F.; Liberty, A.; Foca, M.; Wang, S.; Terry, M.B.; Yin, M.T.; et al. Biomarkers of Aging in HIV-Infected Children on Suppressive Antiretroviral Therapy. JAIDS J. Acquir. Immune Defic. Syndr. 2018, 78, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Saberi, S.; Kalloger, S.E.; Zhu, M.M.T.; Sattha, B.; Maan, E.J.; van Schalkwyk, J.; Money, D.M.; Côté, H.C.F. Dynamics of Leukocyte Telomere Length in Pregnant Women Living with HIV, and HIV-Negative Pregnant Women: A Longitudinal Observational Study. PLoS ONE 2019, 14, e0212273. [Google Scholar] [CrossRef] [PubMed]
- Nagot, N.; Kankasa, C.; Meda, N.; Hofmeyr, J.; Nikodem, C.; Tumwine, J.K.; Karamagi, C.; Sommerfelt, H.; Neveu, D.; Tylleskär, T.; et al. Lopinavir/Ritonavir versus Lamivudine Peri-Exposure Prophylaxis to Prevent HIV-1 Transmission by Breastfeeding: The PROMISE-PEP Trial Protocol ANRS 12174. BMC Infect. Dis. 2012, 12, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagot, N.; Kankasa, C.; Tumwine, J.K.; Meda, N.; Hofmeyr, G.J.; Vallo, R.; Mwiya, M.; Kwagala, M.; Traore, H.; Sunday, A.; et al. Extended Pre-Exposure Prophylaxis with Lopinavir-Ritonavir versus Lamivudine to Prevent HIV-1 Transmission through Breastfeeding up to 50 Weeks in Infants in Africa (ANRS 12174): A Randomised Controlled Trial. Lancet 2016, 387, 566–573. [Google Scholar] [CrossRef]
- Nagot, N.; Singata-Madliki, M.; Cournil, A.; Nalugya, J.; Tassembedo, S.; Quillet, C.; Tonga, M.W.; Tumwine, J.; Meda, N.; Kankasa, C.; et al. Growth, clinical and neurodevelopmental outcomes at school age are similar for children who received 1-year lamivudine or lopinavir/ritonavir HIV prophylaxis in early life. Sci. Rep. 2021, 11, 3173. [Google Scholar] [CrossRef]
- Monnin, A.; Nagot, N.; Peries, M.; Vallo, R.; Meda, N.; Singata-madliki, M.; Tumwine, J.K.; Kankasa, C.; Ngandu, N.; Goga, A.; et al. Mitochondrial DNA Parameters in Blood of Infants Receiving Lopinavir / Ritonavir or Lamivudine Prophylaxis to Prevent Breastfeeding Transmission of HIV-1. J. Clin. Med. 2020, 9, 2972. [Google Scholar] [CrossRef]
- Monnin, A.; Nagot, N.; Eymard-Duvernay, S.; Meda, N.; Tumwine, J.K.; Tylleskär, T.; Van de Perre, P.; Molès, J.-P. Health Outcomes at School Age among Children Who Are HIV-Exposed but Uninfected with Detected Mitochondrial DNA Depletion at One Year. J. Clin. Med. 2020, 9, 3680. [Google Scholar] [CrossRef]
- O’Callaghan, N.J.; Fenech, M. A Quantitative PCR Method for Measuring Absolute Telomere Length. Biol. Proc. Online 2011, 13, 3. [Google Scholar] [CrossRef] [Green Version]
- Fehrer, C.; Voglauer, R.; Wieser, M.; Pfister, G.; Brunauer, R.; Cioca, D.; Grubeck-Loebenstein, B.; Lepperdinger, G. Techniques in Gerontology: Cell Lines as Standards for Telomere Length and Telomerase Activity Assessment. Exp. Gerontol. 2006, 41, 648–651. [Google Scholar] [CrossRef]
- Wedderburn, C.J.; Evans, C.; Yeung, S.; Gibb, D.M.; Donald, K.A.; Prendergast, A.J. Growth and Neurodevelopment of HIV-Exposed Uninfected Children: A Conceptual Framework. Curr. HIV/AIDS Rep. 2019, 16, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Stout, S.A.; Lin, J.; Hernandez, N.; Davis, E.P.; Blackburn, E.; Carroll, J.E.; Glynn, L.M. Validation of Minimally-Invasive Sample Collection Methods for Measurement of Telomere Length. Front. Aging Neurosci. 2017, 9, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanet, D.A.L.; Saberi, S.; Oliveira, L.; Sattha, B.; Gadawski, I.; Côté, H.C.F. Blood and Dried Blood Spot Telomere Length Measurement by QPCR: Assay Considerations. PLoS ONE 2013, 8, e57787. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.L.B.; Richardson, D.S. Sex Differences in Telomeres and Lifespan. Aging Cell 2011, 10, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.; Bann, D.; Wiley, L.; Cooper, R.; Hardy, R.; Nitsch, D.; Martin-Ruiz, C.; Shiels, P.; Sayer, A.A.; Barbieri, M.; et al. Gender and Telomere Length: Systematic Review and Meta-Analysis. Exp. Gerontol. 2014, 51, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Factor-Litvak, P.; Susser, E.; Kezios, K.; McKeague, I.; Kark, J.D.; Hoffman, M.; Kimura, M.; Wapner, R.; Aviv, A. Leukocyte Telomere Length in Newborns: Implications for the Role of Telomeres in Human Disease. Pediatrics 2016, 137, e20153927. [Google Scholar] [CrossRef] [Green Version]
- Wojcicki, J.M.; Olveda, R.; Heyman, M.B.; Elwan, D.; Lin, J.; Blackburn, E.; Epel, E. Cord Blood Telomere Length in Latino Infants: Relation with Maternal Education and Infant Sex. J. Perinatol. 2016, 36, 235–241. [Google Scholar] [CrossRef]
- Okuda, K.; Bardeguez, A.; Gardner, J.P.; Rodriguez, P.; Ganesh, V.; Kimura, M.; Skurnick, J.; Awad, G.; Aviv, A. Telomere Length in the Newborn. Pediatr. Res. 2002, 52, 377–381. [Google Scholar] [CrossRef]
- Aubert, G.; Baerlocher, G.M.; Vulto, I.; Poon, S.S.; Lansdorp, P.M. Collapse of Telomere Homeostasis in Hematopoietic Cells Caused by Heterozygous Mutations in Telomerase Genes. PLoS Genet. 2012, 8, e1002696. [Google Scholar] [CrossRef] [Green Version]
- Hunt, S.C.; Chen, W.; Gardner, J.P.; Kimura, M.; Srinivasan, S.R.; Eckfeldt, J.H.; Berenson, G.S.; Aviv, A. Leukocyte Telomeres Are Longer in African Americans than in Whites: The National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell 2008, 7, 451–458. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, X.; Gutin, B.; Davis, C.L.; Keeton, D.; Thomas, J.; Stallmann-Jorgensen, I.; Mooken, G.; Bundy, V.; Snieder, H.; et al. Leukocyte Telomere Length in Healthy Caucasian and African-American Adolescents: Relationships with Race, Sex, Adiposity, Adipokines, and Physical Activity. J. Pediatr. 2011, 158, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Rewak, M.; Buka, S.; Prescott, J.; De Vivo, I.; Loucks, E.B.; Kawachi, I.; Non, A.L.; Kubzansky, L.D. Race-Related Health Disparities and Biological Aging: Does Rate of Telomere Shortening Differ across Blacks and Whites? Biol. Psychol. 2014, 99, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Hansen, M.E.B.; Hunt, S.C.; Stone, R.C.; Horvath, K.; Herbig, U.; Ranciaro, A.; Hirbo, J.; Beggs, W.; Reiner, A.P.; Wilson, J.G.; et al. Shorter Telomere Length in Europeans than in Africans Due to Polygenetic Adaptation. Hum. Mol. Genet. 2016, 25, 2324–2330. [Google Scholar] [CrossRef] [Green Version]
- Drury, S.S.; Esteves, K.; Hatch, V.; Woodbury, M.; Borne, S.; Adamski, A.; Theall, K.P. Setting the Trajectory: Racial Disparities in Newborn Telomere Length. J. Pediatr. 2015, 166, 1181–1186. [Google Scholar] [CrossRef] [Green Version]
- Nordfjäll, K.; Svenson, U.; Norrback, K.-F.; Adolfsson, R.; Roos, G. Large-Scale Parent-Child Comparison Confirms a Strong Paternal Influence on Telomere Length. Eur. J. Hum. Genet. 2010, 18, 385–389. [Google Scholar] [CrossRef] [Green Version]
- De Meyer, T.; Rietzschel, E.R.; De Buyzere, M.L.; De Bacquer, D.; Van Criekinge, W.; De Backer, G.G.; Gillebert, T.C.; Van Oostveldt, P.; Bekaert, S.; on behalf of the Asklepios Investigators. Paternal Age at Birth Is an Important Determinant of Offspring Telomere Length. Hum. Mol. Genet. 2007, 16, 3097–3102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenberg, D.T.A.; Lee, N.R.; Rej, P.H.; Hayes, M.G.; Kuzawa, C.W. Older Paternal Ages and Grandpaternal Ages at Conception Predict Longer Telomeres in Human Descendants. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190800. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Ye, J.; Li, C.; Zhou, D.; Shen, Q.; Wu, J.; Cao, L.; Wang, T.; Cui, D.; He, S.; et al. Drug Addiction Is Associated with Leukocyte Telomere Length. Sci. Rep. 2013, 3, 1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, G.L.F.; Zeng, H.; Leung, M.-K.; Zhang, H.-J.; Lau, B.W.M.; Liu, Y.-P.; Liu, G.-X.; Sham, P.C.; Chan, C.C.H.; So, K.-F.; et al. Heroin Abuse Accelerates Biological Aging: A Novel Insight from Telomerase and Brain Imaging Interaction. Transl. Psychiatry 2013, 3, e260. [Google Scholar] [CrossRef] [Green Version]
- Levandowski, M.L.; Tractenberg, S.G.; de Azeredo, L.A.; De Nardi, T.; Rovaris, D.L.; Bau, C.H.D.; Rizzo, L.B.; Maurya, P.K.; Brietzke, E.; Tyrka, A.R.; et al. Crack Cocaine Addiction, Early Life Stress and Accelerated Cellular Aging among Women. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 71, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Van de Berg, P.J.E.J.; Griffiths, S.J.; Yong, S.-L.; Macaulay, R.; Bemelman, F.J.; Jackson, S.; Henson, S.M.; ten Berge, I.J.M.; Akbar, A.N.; van Lier, R.A.W. Cytomegalovirus Infection Reduces Telomere Length of the Circulating T Cell Pool. J. Immunol. 2010, 184, 3417–3423. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).