Staphylococcus lugdunensis Bacteremia with an Infected Aortic Thrombus in a Preterm Infant
Abstract
:1. Introduction
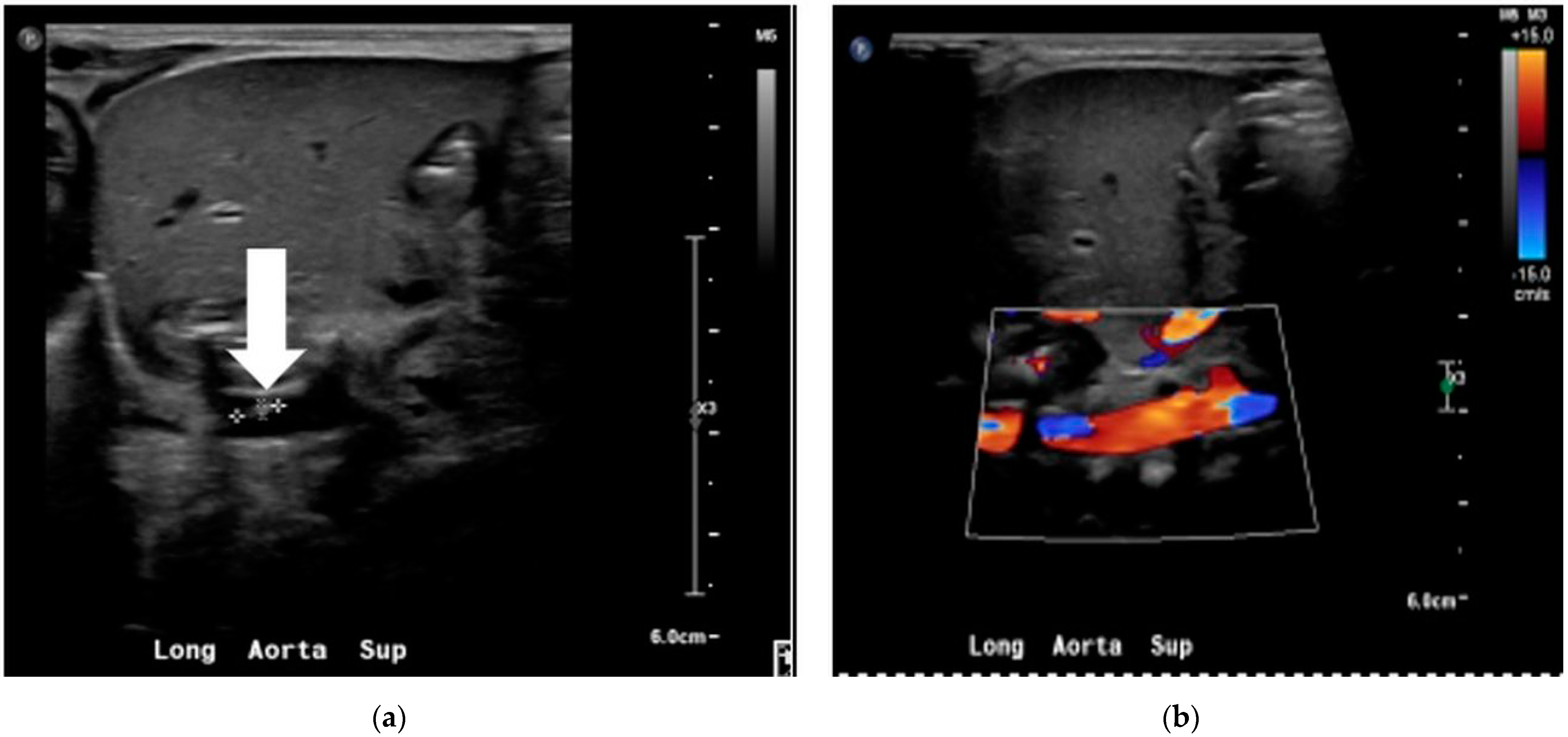
2. Case
2.1. NICU Course
2.2. Staphylococcus lugdunensis Sepsis
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dong, Y.; Speer, C.P. Late-onset neonatal sepsis: Recent developments. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F257–F263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freney, J.; Brun, Y.; Bes, M.; Meugnier, H.; Grimont, F.; Grimont, P.A.D.; Nervi, C.; Fleurette, J. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int. J. Syst. Evol. Microbiol. 1988, 38, 168–172. [Google Scholar] [CrossRef]
- Sotutu, V.; Carapetis, J.; Wilkinson, J.; Davis, A.; Curtis, N. The “surreptitious Staphylococcus”: Staphylococcus lugdunensis endocarditis in a child. Pediatric Infect. Dis. J. 2002, 21, 984–986. [Google Scholar] [CrossRef] [PubMed]
- Böcher, S.; Tønning, B.; Skov, R.L.; Prag, J. Staphylococcus lugdunensis, a common cause of skin and soft tissue infections in the community. J. Clin. Microbiol. 2009, 47, 946–950. [Google Scholar] [CrossRef] [Green Version]
- Garoon, R.B.; Miller, D.; Flynn, H.W., Jr. Acute-onset endophthalmitis caused by Staphylococcus lugdunensis. Am. J. Ophthalmol. Case Rep. 2018, 9, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.P.; Yogev, R.; Shulman, S.T. Staphylococcus lugdunensis: An emerging cause of ventriculoperitoneal shunt infections. Pediatr. Neurosurg. 2001, 35, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, N.; Meilicke, R.; Conrads, G.; Frank, D.; Haase, G. Staphylococcus lugdunensis: Report of a case of peritonitis and an easy-to-perform screening strategy. J. Clin. Microbiol. 1998, 36, 812–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spanu, T.; Rigante, D.; Tamburrini, G.; Fiori, B.; D’Inzeo, T.; Posteraro, B.; Policicchio, D.; Sanguinetti, M.; Fadda, G. Ventriculitis due to Staphylococcus lugdunensis: Two case reports. J. Med. Case Rep. 2008, 2, 267. [Google Scholar] [CrossRef] [PubMed]
- Fleurette, J.; Bès, M.; Brun, Y.; Freney, J.; Forey, F.; Coulet, M.; Reverdy, M.; Etienne, J. Clinical isolates of Staphylococcus lugdunensis and S. schleiferi: Bacteriological characteristics and susceptibility to antimicrobial agents. Res. Microbiol. 1989, 140, 107–118. [Google Scholar] [CrossRef]
- Sato, M.; Kubota, N.; Horiuchi, A.; Kasai, M.; Minami, K.; Matsui, H. Frequency, clinical manifestations, and outcomes of Staphylococcus lugdunensis Bacteremia in children. J. Infect. Chemother. 2016, 22, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Tee, W.S.; Soh, S.Y.; Lin, R.; Loo, L.H. Staphylococcus lugdunensis carrying the mecA gene causes catheter-associated bloodstream infection in premature neonate. J. Clin. Microbiol. 2003, 41, 519–520. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, I.; Hataya, H.; Yamanouchi, H.; Sakakibara, H.; Terakawa, T. Neonatal Staphylococcus lugdunensis urinary tract infection. Pediatr. Int. 2015, 57, 783–785. [Google Scholar] [CrossRef] [PubMed]
- Ittleman, B.R.; Szabo, J.S. Staphylococcus lugdunensis sepsis and endocarditis in a newborn following lotus birth. Cardiol. Young 2018, 28, 1367–1369. [Google Scholar] [CrossRef] [PubMed]
- Frank, K.L.; Del Pozo, J.L.; Patel, R. From clinical microbiology to infection pathogenesis: How daring to be different works for Staphylococcus lugdunensis. Clin. Microbiol. Rev. 2008, 21, 111–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Belkum, A.; Dunne, W.M., Jr. Next-generation antimicrobial susceptibility testing. J. Clin. Microbiol. 2013, 51, 2018–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatzigeorgiou, K.-S.; Sergentanis, T.N.; Tsiodras, S.; Hamodrakas, S.J.; Bagos, P.G. Phoenix 100 versus Vitek 2 in the identification of gram-positive and gram-negative bacteria: A comprehensive meta-analysis. J. Clin. Microbiol. 2011, 49, 3284–3291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef] [Green Version]
- Argemi, X.; Riegel, P.; Lavigne, T.; Lefebvre, N.; Grandpré, N.; Hansmann, Y.; Jaulhac, B.; Prévost, G.; Schramm, F. Implementation of matrix-assisted laser desorption ionization—Time of flight mass spectrometry in routine clinical laboratories improves identification of coagulase-negative staphylococci and reveals the pathogenic role of Staphylococcus lugdunensis. J. Clin. Microbiol. 2015, 53, 2030–2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldman, M.H.; Rasmussen, M.; Olaison, L.; Påhlman, L.I. Endocarditis due to Staphylococcus lugdunensis—A retrospective national registry-based study. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1103–1106. [Google Scholar] [CrossRef] [PubMed]
- Ochi, F.; Tauchi, H.; Kagajo, M.; Murakami, S.; Miyamoto, H.; Hamada, J.; Eguchi-Ishimae, M.; Eguchi, M. Properties of Staphylococcus lugdunensis in Children. Glob. Pediatr. Health 2021, 8, 2333794x211044796. [Google Scholar] [CrossRef]
- Liesenborghs, L.; Peetermans, M.; Claes, J.; Veloso, T.R.; Vandenbriele, C.; Criel, M.; Lox, M.; Peetermans, W.E.; Heilbronner, S.; de Groot, P.G.; et al. Shear-resistant binding to von willebrand factor allows Staphylococcus lugdunensis to adhere to the cardiac valves and initiate endocarditis. J. Infect. Dis. 2016, 213, 1148–1156. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, M.; Bjerketorp, J.; Guss, B.; Frykberg, L. A fibrinogen-binding protein of Staphylococcus lugdunensis. FEMS Microbiol. Lett. 2004, 241, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saracco, P.; Bagna, R.; Gentilomo, C.; Magarotto, M.; Viano, A.; Magnetti, F.; Giordano, P.; Luciani, M.; Molinari, A.C.; Neonatal Working Group of Registro Italiano Trombosi Infantili (RITI); et al. Clinical data of neonatal systemic thrombosis. J. Pediatrics 2016, 171, 60–66.e1. [Google Scholar] [CrossRef]
- Hubbard, E.; Wise, E.; Hubbard, B.; Girard, S.; Kong, B.; Moudgal, V. Tucked away: An infected thrombus. Am. J. Med. 2016, 129, 576–579. [Google Scholar] [CrossRef] [Green Version]
- Beristain-Covarrubias, N.; Perez-Toledo, M.; Thomas, M.R.; Henderson, I.R.; Watson, S.P.; Cunningham, A.F. Understanding infection-induced thrombosis: Lessons learned from animal models. Front. Immunol. 2019, 10, 2569. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Chung, J.W.; Lee, E.J.; Kim, T.H.; Lee, M.S.; Kang, J.M.; Song, E.H.; Jun, J.-B.; Kim, M.-N.; Kim, Y.S.; et al. Incidence, characteristics, and outcomes of Staphylococcus lugdunensis bacteremia. J. Clin. Microbiol. 2010, 48, 3346–3349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soeorg, H.; Huik, K.; Parm, U.; Ilmoja, M.L.; Metelskaja, N.; Metsvaht, T.; Lutsar, I. Genetic relatedness of coagulase-negative Staphylococci from gastrointestinal tract and blood of preterm neonates with late-onset sepsis. Pediatr. Infect. Dis. J. 2013, 32, 389–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoll, B.J.; Hansen, N.; Fanaroff, A.A.; Wright, L.L.; Carlo, W.A.; Ehrenkranz, R.A.; Lemons, J.A.; Donovan, E.F.; Stark, A.R.; Tyson, J.E.; et al. Late-Onset Sepsis in Very Low Birth Weight Neonates: The Experience of the NICHD Neonatal Research Network. Pediatrics 2002, 110, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Letouzey, M.; Foix-L’Hélias, L.; Torchin, H.; Mitha, A.; Morgan, A.S.; Zeitlin, J.; Kayem, G.; Maisonneuve, E.; Delorme, P.; Khoshnood, B.; et al. Cause of preterm birth and late-onset sepsis in very preterm infants: The EPIPAGE-2 cohort study. Pediatr. Res. 2021, 90, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Subbarayan, A.; Colarusso, G.; Hughes, S.M.; Gennery, A.R.; Slatter, M.; Cant, A.J.; Arkwright, P. Clinical features that identify children with primary immunodeficiency diseases. Pediatrics 2011, 127, 810–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Currier, R.; Puck, J.M. SCID newborn screening: What we’ve learned. J. Allergy Clin. Immunol. 2021, 147, 417–426. [Google Scholar] [CrossRef]

| Cell Count | D71 | D72 | D73 |
|---|---|---|---|
| White cell count (×109/L) | 4 | 5.2 | 5.3 |
| Hemoglobin (g/dL) | 10.1 | 13.3 | 11.6 |
| Hematocrit (%) | 32.0 | 39.1 | 34.8 |
| Neutrophil segmented/100 leukocytes (%) | 40 | 40 | 26 |
| Neutrophil—Band/100 leucocytes (%) | 14 | 24 | 14 |
| Lymphocyte (%) | 37 | 21 | 54 |
| Monocyte (%) | 8 | 13 | 6 |
| Eosinophil (%) | 1 | 0 | 0 |
| Metamyelocyte (%) | 0 | 2 | 0 |
| Platelets (×109/L) | 100–149 | 86 | 87 |
| C-reactive protein | 13.52 | 106.57 | 51.25 |
| Blood Culture | Staphylococcus lugdunensis | Staphylococcus spp. | Staphylococcus lugdunensis |
| Urine Culture | No growth | ||
| Cerebrospinal fluid | No growth |
| Staphylococcus lugdunensis | Coagulase-Negative Staphylococcus Species | Staphylococcus lugdunensis | ||||
|---|---|---|---|---|---|---|
| Drug | Susceptibility | MIC | Susceptibility | MIC | Susceptibility | MIC |
| Ampicillin/Sulbactam | R | R | R | |||
| Cephazolin | R | R | R | |||
| Clindamycin | R | ≤0.25 | R | ≥8 | R | ≤0.25 |
| Erythromycin | R | ≥8 | R | ≥8 | R | ≥8 |
| Gentamicin | S | ≤0.5 | R | ≥16 | S | ≤0.5 |
| Levofloxacin | S | ≤0.12 | R | ≥8 | S | 0.25 |
| Linezolid | S | 1 | S | 2 | S | 1 |
| Oxacillin | R | ≥4 | R | ≥4 | R | ≥4 |
| Rifampin | S | ≤0.5 | S | ≤0.5 | S | ≤0.5 |
| Tetracycline | S | ≤1 | S | ≤1 | S | ≤1 |
| Trimethoprim/ Sulfamethoxazole | S | ≤10 | R | ≥320 | S | ≤10 |
| Vancomycin | S | ≤0.5 | S | 1 | S | ≤0.5 |
| Year of Report | GA | B.WT. | AGE | Clinical Presentation | Comorbidity | Antibiotic | Central Line | Recovery |
|---|---|---|---|---|---|---|---|---|
| 2003 [12] | 29 w | 980 g | 23 d | Bacteremia—Apnea | RDS, PDA and pulmonary hemorrhage | Vancomycin | Yes | Yes |
| 2015 [13] | 41 + 4/7 w | 3284 g | 18d | UTI -Fever with rash | none | Cefazolin | No | Yes |
| 2018 [14] | Unknown | NA | 1 d | Infective endocarditis | PROM | Nafcillin | No | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mani, S.; Chandrasekharan, P. Staphylococcus lugdunensis Bacteremia with an Infected Aortic Thrombus in a Preterm Infant. Children 2022, 9, 46. https://doi.org/10.3390/children9010046
Mani S, Chandrasekharan P. Staphylococcus lugdunensis Bacteremia with an Infected Aortic Thrombus in a Preterm Infant. Children. 2022; 9(1):46. https://doi.org/10.3390/children9010046
Chicago/Turabian StyleMani, Srinivasan, and Praveen Chandrasekharan. 2022. "Staphylococcus lugdunensis Bacteremia with an Infected Aortic Thrombus in a Preterm Infant" Children 9, no. 1: 46. https://doi.org/10.3390/children9010046






