Partial Obstruction of Ventricular Catheters Affects Performance in a New Catheter Obstruction Model of Hydrocephalus
Abstract
:1. Introduction
2. Methods
2.1. Catheter Occlusion Assay
2.2. 3D Printing of the Ventricle Phantom
2.3. Assessment of Catheter Pressure and Flow
2.4. Statistical Analysis
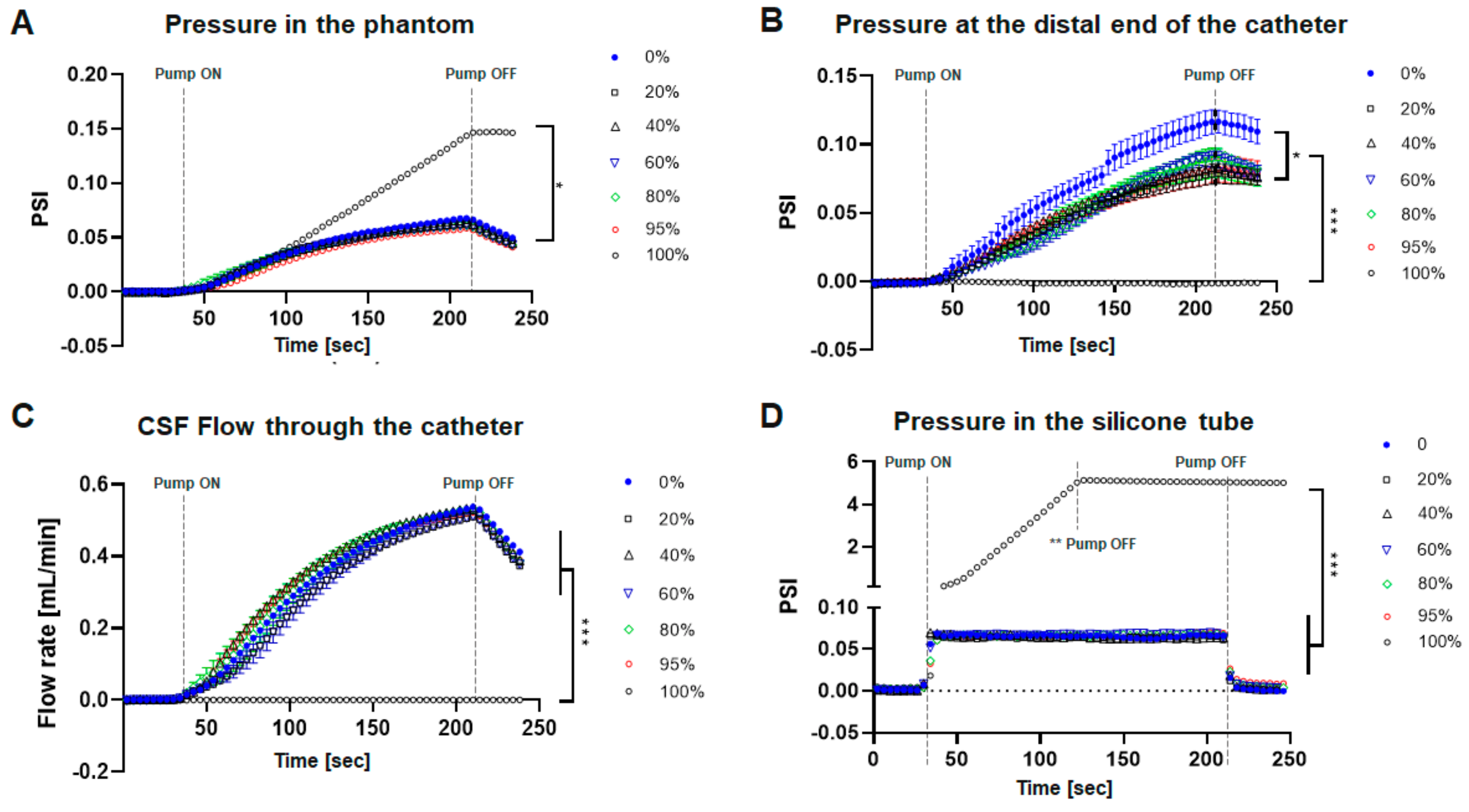
3. Results
Catheter Performance under Obstructive Conditions
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Isaacs, A.M.; Riva-Cambrin, J.; Yavin, D.; Hockley, A.; Pringsheim, T.M.; Jette, N.; Lethebe, B.C.; Lowerison, M.; Dronyk, J.; Hamilton, M.G. Age-specific global epidemiology of hydrocephalus: Systematic review, metanalysis and global birth surveillance. PLoS ONE 2018, 13, e0204926. [Google Scholar] [CrossRef] [PubMed]
- Rekate, H.L. A contemporary definition and classification of hydrocephalus. Semin. Pediatr. Neurol. 2009, 16, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.J.; Walker, C.T.; Jacobson, M.; Phillips, V.; Silberstein, H.J. Revision rate of pediatric ventriculoperitoneal shunts after 15 years. J. Neurosurg. Pediatr. 2013, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.V.; Riva-Cambrin, J.; Butler, J.; Browd, S.R.; Drake, J.M.; Holubkov, R.; Kestle, J.R.W.; Limbrick, D.D.; Simon, T.D.; Tamber, M.S.; et al. Outcomes of CSF shunting in children: Comparison of Hydrocephalus Clinical Research Network cohort with historical controls. J. Neurosurg. Pediatr. 2013, 12, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Hanak, B.W.; Bonow, R.H.; Harris, C.A.; Browd, S.R. Cerebrospinal Fluid Shunting Complications in Children. Pediatr. Neurosurg. 2017, 52, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Hanak, B.W.; Ross, E.F.; Harris, C.A.; Browd, S.R.; Shain, W. Toward a better understanding of the cellular basis for cerebrospinal fluid shunt obstruction: Report on the construction of a bank of explanted hydrocephalus devices. J. Neurosurg. Pediatr. 2016, 18, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, P.; Sondheimer, J.; Petroj, A.; Gluski, J.; Jea, A.; Whitehead, W.E.; Sood, S.; Ham, S.D.; Rocque, B.G.; Marupudi, N.I.; et al. A multicenter retrospective study of heterogeneous tissue aggregates obstructing ventricular catheters explanted from patients with hydrocephalus. Fluids Barriers CNS 2021, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Hanak, B.W.; Hsieh, C.Y.; Donaldson, W.; Browd, S.R.; Lau, K.K.; Shain, W. Reduced cell attachment to poly(2-hydroxyethyl methacrylate)-coated ventricular catheters in vitro. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.A.; McAllister, J.P., 2nd. What we should know about the cellular and tissue response causing catheter obstruction in the treatment of hydrocephalus. Neurosurgery 2012, 70, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Khodadadei, F.; Liu, A.P.; Harris, C.A. A high-resolution real-time quantification of astrocyte cytokine secretion under shear stress for investigating hydrocephalus shunt failure. Commun. Biol. 2021, 4, 387. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.A.; Resau, J.H.; Hudson, E.A.; West, R.A.; Moon, C.; McAllister, J.P., II. Mechanical contributions to astrocyte adhesion using a novel in vitro model of catheter obstruction. Exp. Neurol. 2010, 222, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.A.; Resau, J.H.; Hudson, E.A.; West, R.A.; Moon, C.; Black, A.D.; McAllister, J.P. Effects of surface wettability, flow, and protein concentration on macrophage and astrocyte adhesion in an in vitro model of central nervous system catheter obstruction. J. Biomed. Mater. Res. Part A 2011, 97, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.; Pearson, K.; Hadley, K.; Zhu, S.; Browd, S.; Hanak, B.W.; Shain, W. Fabrication of three-dimensional hydrogel scaffolds for modeling shunt failure by tissue obstruction in hydrocephalus. Fluids Barriers CNS 2015, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kwok, N.; Holsapple, J.; Heldt, T.; Bourouiba, L. Enhanced wall shear stress prevents obstruction by astrocytes in ventricular catheters. J. R. Soc. Interface 2020, 17, 20190884. [Google Scholar] [CrossRef] [PubMed]
- Devathasan, D.; Bentley, R.T.; Enriquez, A.; Yang, Q.; Thomovsky, S.A.; Thompson, C.; Lee, A.E.; Lee, H. Development of an In Vitro Hemorrhagic Hydrocephalus Model for Functional Evaluation of Magnetic Microactuators Against Shunt Obstructions. World Neurosurg. 2021, 155, e294–e300. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Morris, M.; Olivero, W.; Boop, F.; Sanford, R.A. Computational and experimental study of proximal flow in ventricular catheters. J. Neurosurg. 2003, 99, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Galarza, M.; Giménez, Á.; Pellicer, O.; Valero, J.; Amigó, J.M. New designs of ventricular catheters for hydrocephalus by 3-D computational fluid dynamics. Child’s Nerv. Syst. 2015, 31, 37–48. [Google Scholar] [CrossRef] [PubMed]
- TerMaath, S.; Stefanski, D.; Killeffer, J. Computational Modeling and Simulation to Quantify the Effects of Obstructions on the Performance of Ventricular Catheters Used in Hydrocephalus Treatment. In Biomedical Engineering Technologies; Humana: New York, NY, USA, 2022; pp. 767–786. [Google Scholar]
- Ginsberg, H.J.; Sum, A.; Drake, J.M.; Cobbold, R.S. Ventriculoperitoneal shunt flow dependency on the number of patent holes in a ventricular catheter. Pediatr. Neurosurg. 2000, 33, 7–11. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Vinzani, M.; Romero, B.; Chan, A.Y.; Castañeyra-Ruiz, L.; Muhonen, M. Partial Obstruction of Ventricular Catheters Affects Performance in a New Catheter Obstruction Model of Hydrocephalus. Children 2022, 9, 1453. https://doi.org/10.3390/children9101453
Lee S, Vinzani M, Romero B, Chan AY, Castañeyra-Ruiz L, Muhonen M. Partial Obstruction of Ventricular Catheters Affects Performance in a New Catheter Obstruction Model of Hydrocephalus. Children. 2022; 9(10):1453. https://doi.org/10.3390/children9101453
Chicago/Turabian StyleLee, Seunghyun, Michael Vinzani, Bianca Romero, Alvin Y. Chan, Leandro Castañeyra-Ruiz, and Michael Muhonen. 2022. "Partial Obstruction of Ventricular Catheters Affects Performance in a New Catheter Obstruction Model of Hydrocephalus" Children 9, no. 10: 1453. https://doi.org/10.3390/children9101453




