Conjoined Twins Complicating a Dichorionic Triplet Pregnancy after Intracytoplasmic Sperm Injection: A Case Report and Review of the Literature
Abstract
1. Introduction
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osterman, M.J.K.; Hamilton, B.E.; Martin, J.A.; Driscoll, A.K.; Valenzuela, C.P. Births: Final Data for 2020 Figure 1. Live Births and General Fertility Rates: United States. Natl. Vital Stat. Rep. 2022, 70, 8–9. [Google Scholar]
- Mutchinick, O.M.; Luna-Muñoz, L.; Amar, E.; Bakker, M.K.; Clementi, M.; Cocchi, G.; da Graça Dutra, M.; Feldkamp, M.L.; Landau, D.; Leoncini, E.; et al. Conjoined Twins: A Worldwide Collaborative Epidemiological Study of the International Clearinghouse for Birth Defects Surveillance and Research. Am. J. Med. Genet. Part C Semin. Med. Genet. 2011, 157, 274–287. [Google Scholar] [CrossRef]
- Aparna, C.; Renuka, I.V.; Sailabala, G.; Nayudamma, Y. Dicephalus Dipus Tribrachius: A Case Report of Unusual Conjoined Twins. Indian J. Pathol. Microbiol. 2010, 53, 814. [Google Scholar] [CrossRef]
- El Khoury, R.S.; Azar, G.B. The Ultrasonographic Prenatal Diagnosis of Conjoined Twins. A Case Report. J. Med. Liban 2010, 58, 18–20. [Google Scholar]
- Sebire, N.; Sepulveda, W.; Jeanty, P.; Nyberg, D.; Nicolaides, K. Multiple Gestations. In Diagnostic Imaging of Fetal Anomalies; Nyberg, D., McGahan, J., Pretorius, D., Pilu, G., Eds.; Lippincott Williams & Wilkins: Philadellphia, PA, USA, 2003; pp. 777–813. [Google Scholar]
- Cunningham, F.G.; Leveno, K.J.; Bloom, S.L.; Dashe, J.S.; Hoffman, B.L.; Casey, B.M.; Spong, C.Y. (Eds.) Multifetal Pregnancy. In Williams Obstetrics; McGraw Hill Medical: New York, NY, USA, 2018; pp. 863–897. ISBN 9781259644320. [Google Scholar]
- Mendilcioglu, I.; Simsek, M. Conjoined Twins in a Trichorionic Quadruplet Pregnancy after Ovulation Induction with Clomiphene Citrate. Fetal Diagn. Ther. 2008, 24, 51–54. [Google Scholar] [CrossRef]
- Sepulveda, W.; Munoz, H.; Alcalde, J.L. Conjoined Twins in a Triplet Pregnancy: Early Prenatal Diagnosis with Three-Dimensional Ultrasound and Review of the Literature. Ultrasound Obstet. Gynecol. 2003, 22, 199–204. [Google Scholar] [CrossRef]
- Pajkrt, E.; Jauniaux, E. First-Trimester Diagnosis of Conjoined Twins. Prenat. Diagn. 2005, 25, 820–826. [Google Scholar] [CrossRef]
- Tonni, G.; Ventura, A.; Vito, I.; Piana, D.; Bonasoni, M.P.; De Felice, C. Cephalothoracopagus, Janiceps, Disymmetros, Monoomphalian Conjoined Twins Undiagnosed until Early Second Trimester. Arch. Gynecol. Obstet. 2011, 283, 387–390. [Google Scholar] [CrossRef]
- Winkler, N.; Kennedy, A.; Byrne, J.; Woodward, P. The Imaging Spectrum of Conjoined Twins. Ultrasound Q. 2008, 24, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.T.; Weinberg, P.M.; Gruber, P.J.; Sutton, M.G.S.J. Conjoined Hearts in Thoracopagus Twins. Pediatr. Cardiol. 2011, 33, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.L.; Tock, E.P.C.; Dawood, M.Y.; Ratnam, S.S. Conoined Twins in a Triplet Pregnancy. Am. J. Dis. Child. 1971, 122, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, Y.; Ben-Shlomo, I.; Weiner, E.; Shalev, E. First Trimester Diagnosis of Conjoined Twins in a Triplet Pregnancy after IVF and ICSI: Case Report. Hum. Reprod. 2000, 15, 1413–1415. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Evans, M.I.; Goldberg, J.D.; Horenstein, J.; Wapner, R.J.; Ayoub, M.A.; Stone, J.; Lipitz, S.; Achiron, R.; Holzgreve, W.; Brambati, B.; et al. Selective Termination for Structural, Chromosomal, and Mendelian Anomalies: International Experience. Am. J. Obstet. Gynecol. 1999, 181, 893–897. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Wu, L.P.; Wu, L.J.; Chen, G.Z.; Sun, K.; Zhang, Z.F.; Shen, R. Echocardiographic Assessment of Conjoined Twins with Congenital Heart Disease in Shanghai. Echocardiography 2009, 26, 691–698. [Google Scholar] [CrossRef]
- Spencer, R. Anatomic Description of Conjoined Twins: A Plea for Standardized Terminology. J. Pediatr. Surg. 1996, 31, 941–944. [Google Scholar] [CrossRef]
- Berkowitz, R.L.; Lynch, L.; Chitkara, U.; Wilkins, I.A.; Mehalek, K.E.; Alvarez, E. Selective Reduction of Multifetal Pregnancies in the First Trimester. N. Engl. J. Med. 1988, 318, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Wapner, R.J.; Davis, G.H.; Johnson, A.; Weinblatt, V.J.; Fischer, R.L.; Jackson, L.J.; Chervenak, F.A.; McCullough, L.B. Selective Reduction of Multifetal Pregnancies. Lancet 1990, 335, 90–93. [Google Scholar] [CrossRef]
- Lipitz, S.; Ravia, J.; Zolti, M.; Achiron, R.; Wolf, Y.; Kazanstein, A.; Goldenberg, M.; Seidman, D.S. Sequential Genetic Events Leading to Conjoined Twins in a Monozygotic Triplet Pregnancy. Hum. Reprod. 1995, 10, 3130–3132. [Google Scholar] [CrossRef]
- Shepherd, L.J.; Smith, G.N. Conjoined Twins in a Triplet Pregnancy: A Case Report. Case Rep. Obstet. Gynecol. 2011, 2011, 235873. [Google Scholar] [CrossRef]
- Rohilla, S.; Dahiya, K.; Rathee, S.; Yadav, R.K.; Dhaulakhandi, D.B. Conjoined Twins in a Spontaneous Trichorionic Quadruplet Pregnancy: A Case Report. J. Reprod. Med. 2011, 56, 351–355. [Google Scholar]
- Huisman, T.A.G.M.; Arulrajah, S.; Meuli, M.; Brehmer, U.; Beinder, E. Fetal MRI of Conjoined Twins Who Switched Their Relative Positions. Pediatr. Radiol. 2009, 40, 353–357. [Google Scholar] [CrossRef]
- Bonilla-Musoles, F.; Raga, F.; Bonilla, F.; Blanes, J.; Osborne, N.G. Early Diagnosis of Conjoined Twins Using Two-Dimensional Color Doppler and Three-Dimensional Ultrasound. J. Natl. Med. Assoc. 1998, 90, 552. [Google Scholar] [PubMed]
- Reddy, U.M.; Filly, R.A.; Copel, J.A. Prenatal Imaging: Ultrasonography and Magnetic Resonance Imaging. Obstet. Gynecol. 2008, 112, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Wenstrom, K.D.; Syrop, C.H.; Hammitt, D.G.; Van Voorhis, B.J. Increased Risk of Monochorionic Twinning Associated with Assisted Reproduction. Fertil. Steril. 1993, 60, 510–514. [Google Scholar] [CrossRef]
- Poret, H.; Blanchard, M.; Lemseffer, M.; Royere, D.; Guerif, F. Conjoined Twins after Intracytoplasmic Sperm Injection and Transfer of a Single Day 2 Embryo: Case Report. Fertil. Steril. 2010, 93, 268.e7–268.e9. [Google Scholar] [CrossRef] [PubMed]
- D’Alton, M.E.; Dudley, D.K. The Ultrasonographic Prediction of Chorionicity in Twin Gestation. Am. J. Obstet. Gynecol. 1989, 160, 557–561. [Google Scholar] [CrossRef]
- Wood, S.L.; St. Onge, R.; Connors, G.; Elliot, P.D. Evaluation of the Twin Peak or Lambda Sign in Determining Chorionicity in Multiple Pregnancy. Obstet. Gynecol. 1996, 88, 6–9. [Google Scholar] [CrossRef]
- Milki, A.A.; Jun, S.H.; Hinckley, M.D.; Behr, B.; Giudice, L.C.; Westphal, L.M. Incidence of Monozygotic Twinning with Blastocyst Transfer Compared to Cleavage-Stage Transfer. Fertil. Steril. 2003, 79, 503–506. [Google Scholar] [CrossRef]
- Sheiner, E.; Har-Vardi, I.; Potashnik, G. The Potential Association between Blastocyst Transfer and Monozygotic Twinning. Fertil. Steril. 2001, 75, 217–218. [Google Scholar] [CrossRef]
- Hirata, T.; Osuga, Y.; Fujimoto, A.; Oishi, H.; Hiroi, H.; Fujiwara, T.; Yano, T.; Taketani, Y. Conjoined Twins in a Triplet Pregnancy after Intracytoplasmic Sperm Injection and Blastocyst Transfer: Case Report and Review of the Literature. Fertil. Steril. 2009, 91, 933.e9–933.e12. [Google Scholar] [CrossRef]
- Hershlag, A.; Paine, T.; Cooper, G.W.; Scholl, G.M.; Rawlinson, K.; Kvapil, G. Monozygotic Twinning Associated with Mechanical Assisted Hatching. Fertil. Steril. 1999, 71, 144–146. [Google Scholar] [CrossRef]
- Abusheika, N.; Salha, O.; Sharma, V.; Brinsden, P. Monozygotic Twinning and IVF/ICSI Treatment: A Report of 11 Cases and Review of Literature. Hum. Reprod. Update 2000, 6, 396–403. [Google Scholar] [CrossRef]
- Tarlatzis, B.C.; Qublan, H.S.; Sanopoulou, T.; Zepiridis, L.; Grimbizis, G.; Bontis, J. Increase in the Monozygotic Twinning Rate after Intracytoplasmic Sperm Injection and Blastocyst Stage Embryo Transfer. Fertil. Steril. 2002, 77, 196–198. [Google Scholar] [CrossRef]
- Edwards, R.G.; Mettler, L.; Walters, D.E. Identical Twins and in Vitro Fertilization. J. In Vitro Fert. Embryo Transf. 1986, 3, 114–117. [Google Scholar] [CrossRef]
- Cohen, J.; Elsner, C.; Kort, H.; Malter, H.; Massey, J.; Mayer, M.P.; Wiemer, K. Impairment of the Hatching Process following IVF in the Human and Improvement of Implantation by Assisting Hatching Using Micromanipulation. Hum. Reprod. 1990, 5, 7–13. [Google Scholar] [CrossRef]
- Mercan, R.; Oktem, O.; Salar, Z.; Nuhoglu, A.; Balaban, B.; Urman, B. Conjoined Twins after Intracytoplasmic Sperm Injection and Transfer of Day-3 Embryos. Fertil. Steril. 2011, 96, e111–e114. [Google Scholar] [CrossRef]
- Logroño, R.; Garcia-Lithgow, C.; Harris, C.; Kent, M.; Meisner, L. Heteropagus Conjoined Twins Due to Fusion of Two Embryos: Report and Review—PubMed. Am. J. Med. Genet. 1997, 73, 239–243. [Google Scholar] [CrossRef]
- Skupski, D.W.; Streltzoff, J.; Hutson, J.M.; Rosenwaks, Z.; Cohen, J.; Chervenak, F.A. Early Diagnosis of Conjoined Twins in Triplet Pregnancy after in Vitro Fertilization and Assisted Hatching. J. Ultrasound Med. 1995, 14, 611–615. [Google Scholar] [CrossRef]
- Timor-Tritsch, I.E.; Monteagudo, A.; Horan, C.; Stangel, D.J.J. Dichorionic Triplet Pregnancy with the Monoamniotic Twin Pair Concordant for Omphalocele and Bladder Exstrophy. Ultrasound Obstet. Gynecol. 2000, 16, 669–671. [Google Scholar] [CrossRef]
- Charles, A.; Dickinson, J.E.; Watson, S.; Phillips, N.; Yovich, J. Diamniotic Conjoined Fetuses in a Triplet Pregnancy: An Insight into Embryonic Topology: Pediatr. Dev. Pathol. 2005, 8, 666–672. [Google Scholar] [CrossRef]
- Talebian, M.; Rahimi-Sharbaf, F.; Shirazi, M.; Teimoori, B.; Izadi-mood, N.; Sarmadi, S. Conjoined Twins in a Monochorionic Triplet Pregnancy after in Vitro Fertilization: A Case Report. Iran. J. Reprod. Med. 2015, 13, 729. [Google Scholar] [PubMed]
- Castro, P.T.; Werner, H.; Araujo Júnior, E. First-Trimester Diagnosis of Conjoined Twins in a Multifetal Pregnancy after Assisted Reproduction Technique Using HDlive Rendering. J. Ultrasound 2017, 20, 85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, H.; Zhou, Q.; Li, J.; Zeng, S. Triplet Pregnancy from the Transfer of Two Blastocysts Demonstrating a Twin Reversed Arterial Perfusion Sequence with a Conjoined-Twins Pump Fetus. Int. J. Gynecol. Obstet. 2017, 137, 196–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Deng, C.; Hu, Q.; Liao, H.; Wang, X.; Yu, H. Conjoined Twins in Dichorionic Diamniotic Triplet Pregnancy: A Report of Three Cases and Literature Review. BMC Pregnancy Childbirth 2021, 21, 687. [Google Scholar] [CrossRef]
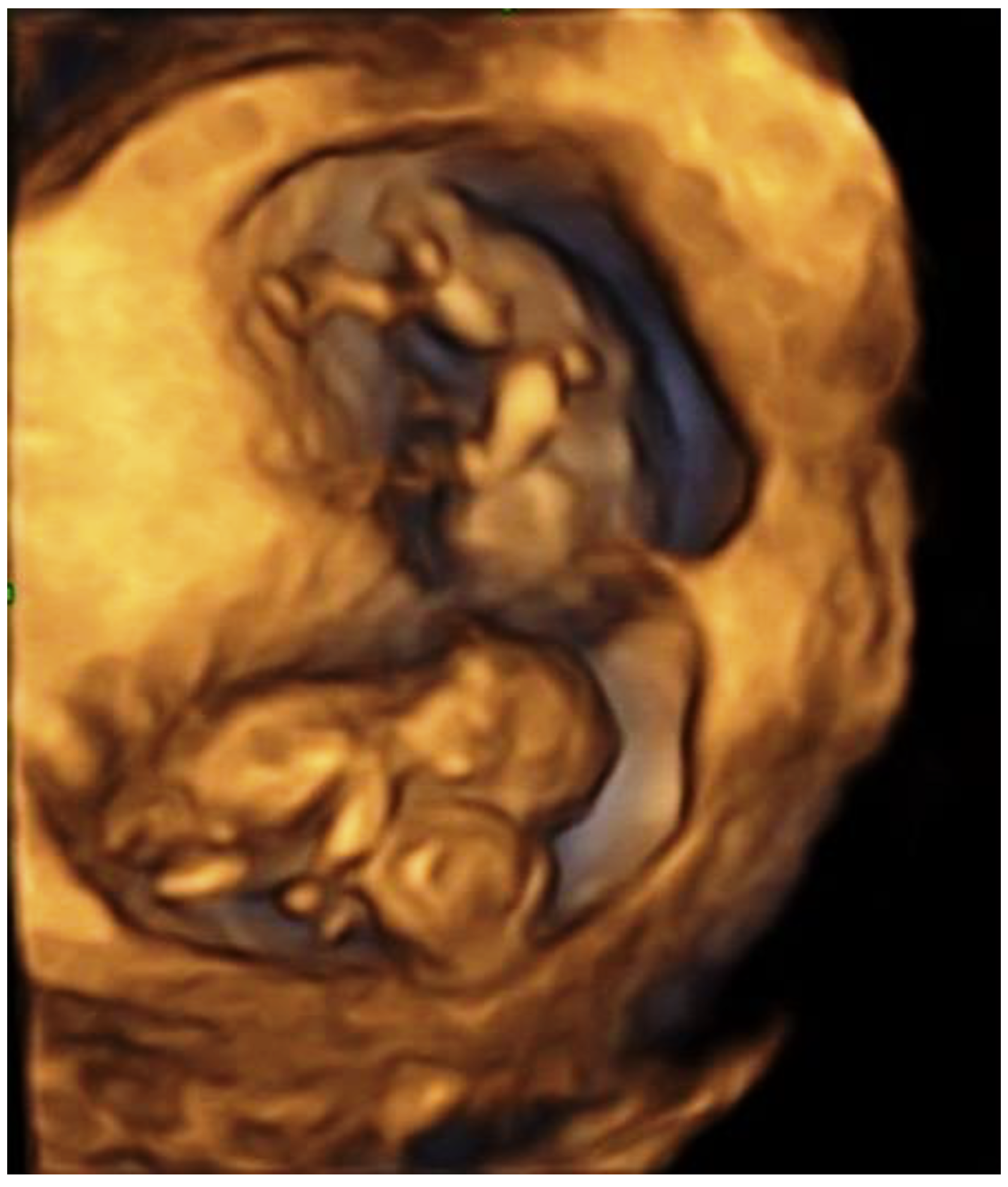
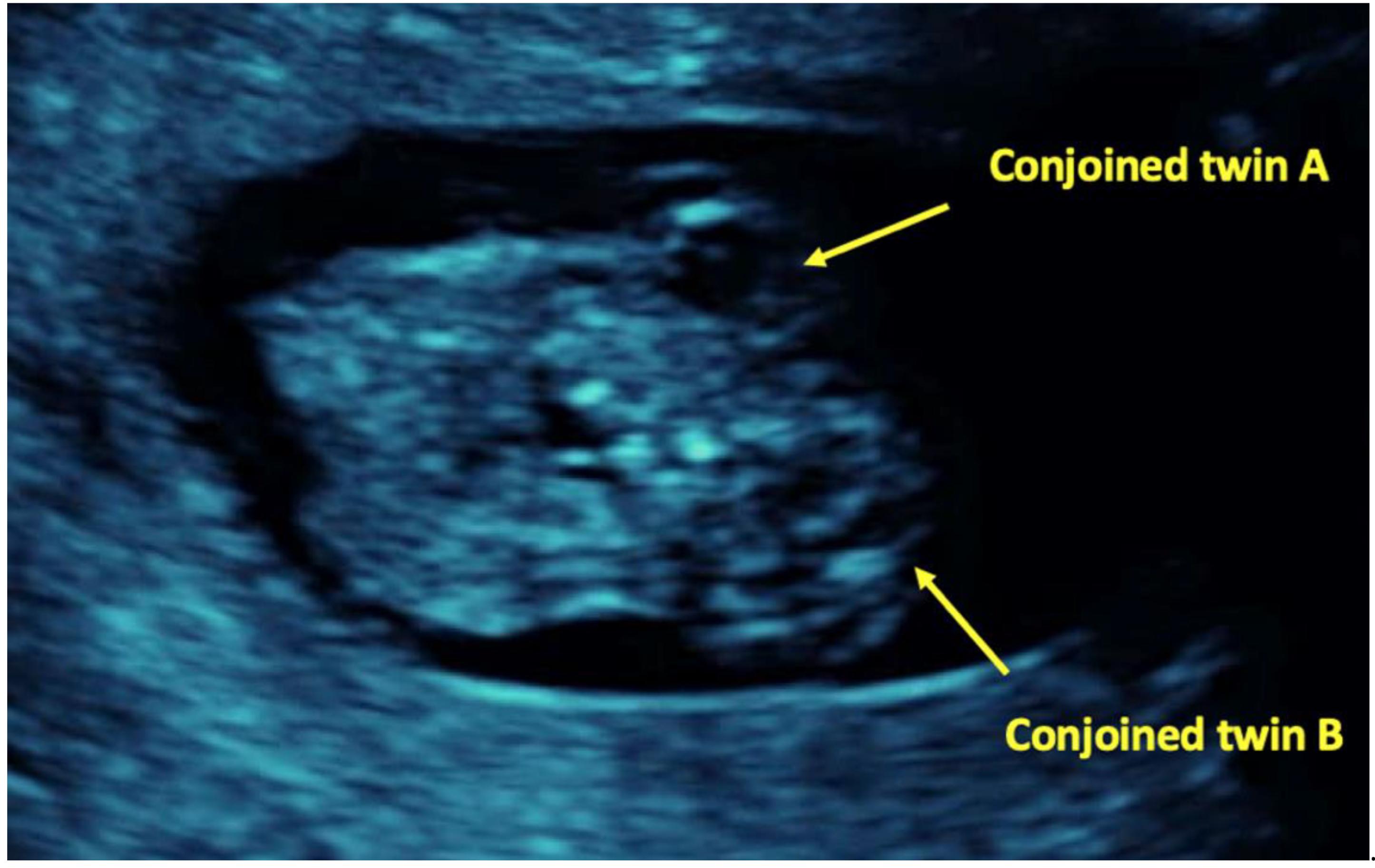


| Case No. | Authors | Country | Age | Gravity | Parity | Treatment | Triplet Type | Type of Conjoining | Gestational Age at Diagnosis | Selective Termination | Result | No. of Newborns |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Skupski et al., 1995 [40] | USA | 35 | NS | 2 | IVF | DCDA | Thoraco-omphalopagus | 12 weeks | + | Ongoing pregnancy | NA |
| 2 | Goldberg et al., 2000 [14] | Israel | 28 | 1 | 0 | ICSI | DCDA | Thoraco-omphalopagus | 8 weeks 4 days | + | Ongoing pregnancy | NA |
| 3 | Timor-Tritsch et al., 2000 [41] | USA | NS | NS | NS | IVF | DCDA | Omphalopagus | 10 weeks | + | NS | 1 |
| 4 | Charles et al., 2005 [42] | Australia | 20 | NS | NS | IVF | DCDA | Omphalopagus | 10 weeks | + | Death due to premature delivery at 21 weeks of gestation | 0 |
| 5 | Hirata et al., 2009 [32] | Japan | 34 | 1 | 1 | ICSI | DCDA | Thoracopagus | 8 weeks | - | Spontaneous cardiac arrest of the CTs at 10 weeks and 3 days of gestation A male neonate weighing 2792g was born at 39 weeks of gestation | 1 |
| 6 | Shepherd et al., 2011 [21] | Canada | 32 | 1 | 0 | Ovulation Induction | DCDA | Thoraco-omphalopagus | 13 weeks 1 day | + | A male neonate weighing 3590g was delivered vaginally at 40 of weeks gestation | 1 |
| 7 | Talebian et al., 2015 [43] | Iran | 38 | 1 | 0 | ICSI | MCDA | Thoraco-omphalopagus | 12 weeks 2 days | + | Selective fetocide performed at 16 weeks of gestation All fetuses were spontaneously aborted | 0 |
| 8 | Castro et al., 2017 [44] | Brazil | 32 | NS | NS | ICSI | DCDA | Thoraco-omphalopagus | 9 weeks | - | Spontaneous cardiac arrest of the CTs at 13 weeks of gestation A female neonate weighing 2750g was vaginally delivered at 38 weeks | 1 |
| 9 | Yuan et al., 2017 [45] | China | 39 | 3 | 0 | IVF | MCDA | Thoraco-omphalopagus | 10 weeks | - | Termination of pregnancy by induced abortion | 0 |
| 10 | Liu et al.,.2021 [46] | China | 22 | 1 | 0 | IVF | DCDA | Thoraco-omphalopagus | 13 weeks 5 days | + | A female neonate weighing 2760g was delivered by caesarean section at 37 weeks and 4 days of gestation | 1 |
| 11 | Eleftheriades et al., 2022 (our case) | Greece | 44 | 1 | 0 | ICSI | DCDA | Thoraco-omphalopagus | 11 weeks | + | A healthy neonate weighting 2200g was delivered by caesarean section at 38 weeks and 1 day of gestation | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eleftheriades, A.; Christopoulos, P.; Tsapakis, E.; Tsarna, E.; Vlahos, N.F.; Kalampokas, E.; Bolla, D.; Eleftheriades, M. Conjoined Twins Complicating a Dichorionic Triplet Pregnancy after Intracytoplasmic Sperm Injection: A Case Report and Review of the Literature. Children 2022, 9, 1549. https://doi.org/10.3390/children9101549
Eleftheriades A, Christopoulos P, Tsapakis E, Tsarna E, Vlahos NF, Kalampokas E, Bolla D, Eleftheriades M. Conjoined Twins Complicating a Dichorionic Triplet Pregnancy after Intracytoplasmic Sperm Injection: A Case Report and Review of the Literature. Children. 2022; 9(10):1549. https://doi.org/10.3390/children9101549
Chicago/Turabian StyleEleftheriades, Anna, Panagiotis Christopoulos, Elsa Tsapakis, Ermioni Tsarna, Nikolaos F. Vlahos, Emmanouil Kalampokas, Daniele Bolla, and Makarios Eleftheriades. 2022. "Conjoined Twins Complicating a Dichorionic Triplet Pregnancy after Intracytoplasmic Sperm Injection: A Case Report and Review of the Literature" Children 9, no. 10: 1549. https://doi.org/10.3390/children9101549
APA StyleEleftheriades, A., Christopoulos, P., Tsapakis, E., Tsarna, E., Vlahos, N. F., Kalampokas, E., Bolla, D., & Eleftheriades, M. (2022). Conjoined Twins Complicating a Dichorionic Triplet Pregnancy after Intracytoplasmic Sperm Injection: A Case Report and Review of the Literature. Children, 9(10), 1549. https://doi.org/10.3390/children9101549










