The Joint Effect of Perceived Psychosocial Stress and Phthalate Exposure on Hormonal Concentrations during the Early Stage of Pregnancy: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. The Questionnaire Method of Data Collection
2.3. Qualitative and Quantitative Analysis of Phthalate Metabolites from Urine Spots
2.4. Qualitative and Quantitative Analysis of Maternal Hormonal Concentrations from Blood Serum Spots
2.5. Statistics
3. Results
3.1. Demographic Characteristics
3.2. Biomonitoring of Phthalate Metabolites
3.3. Analyses of Hormones
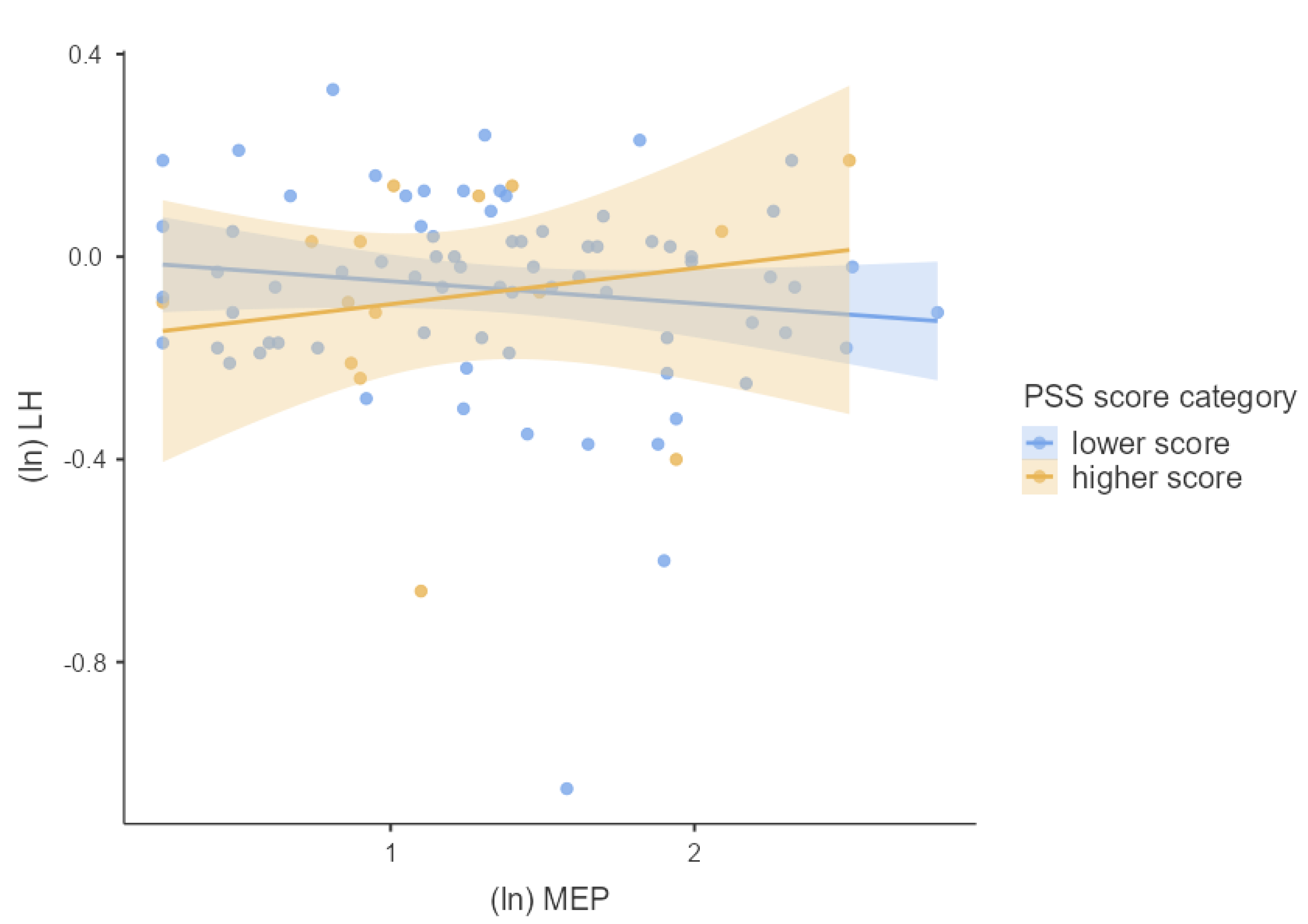
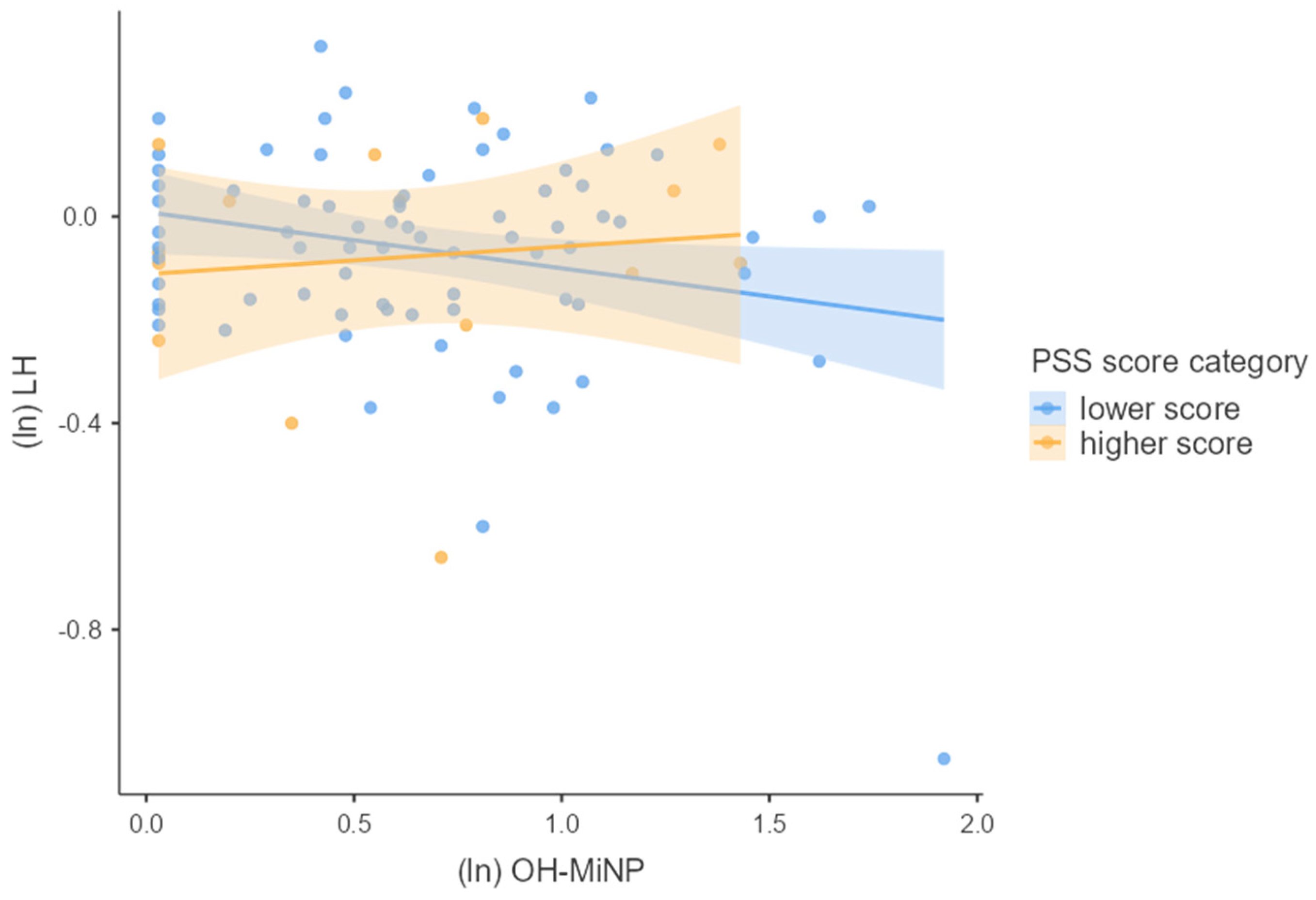
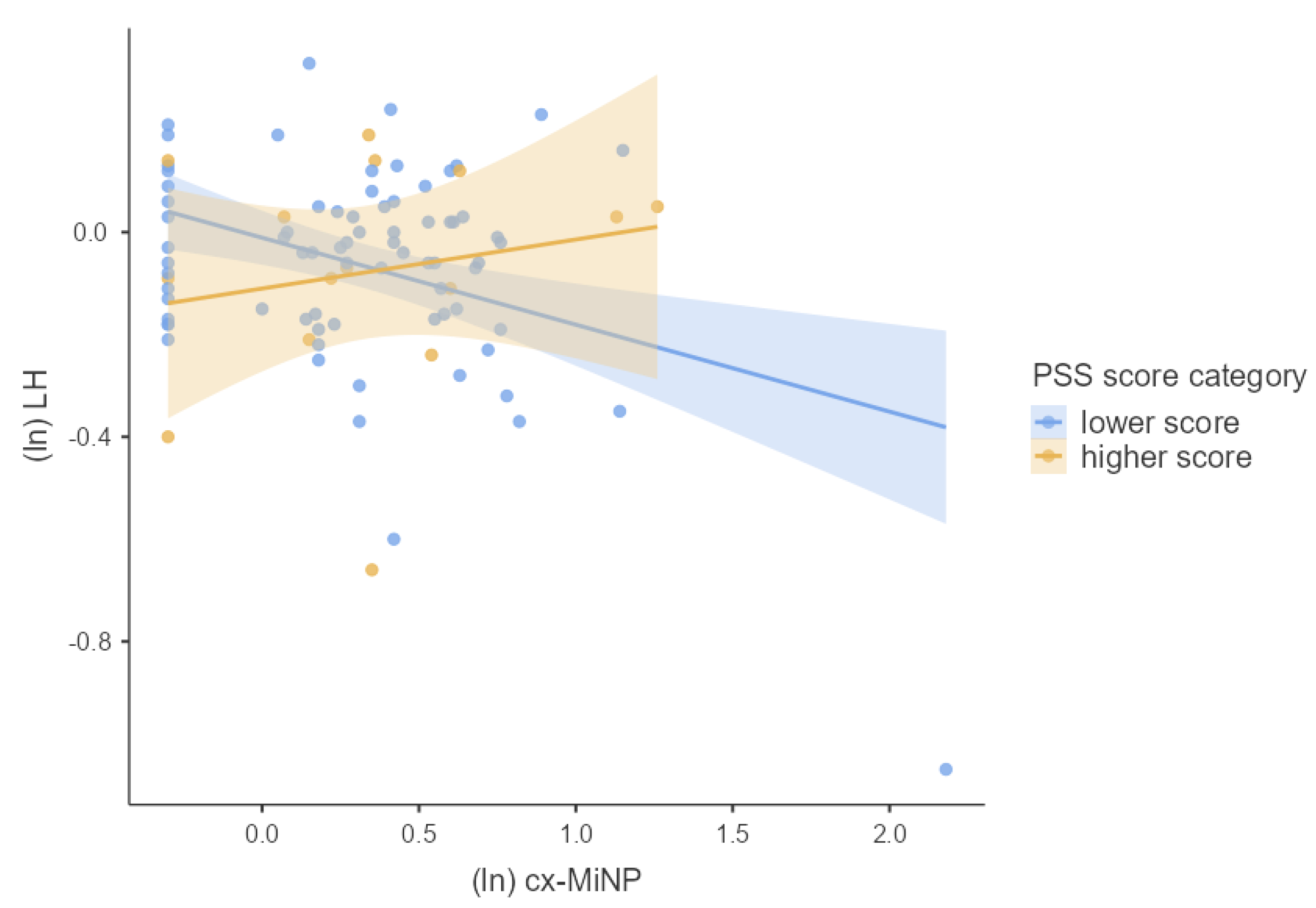
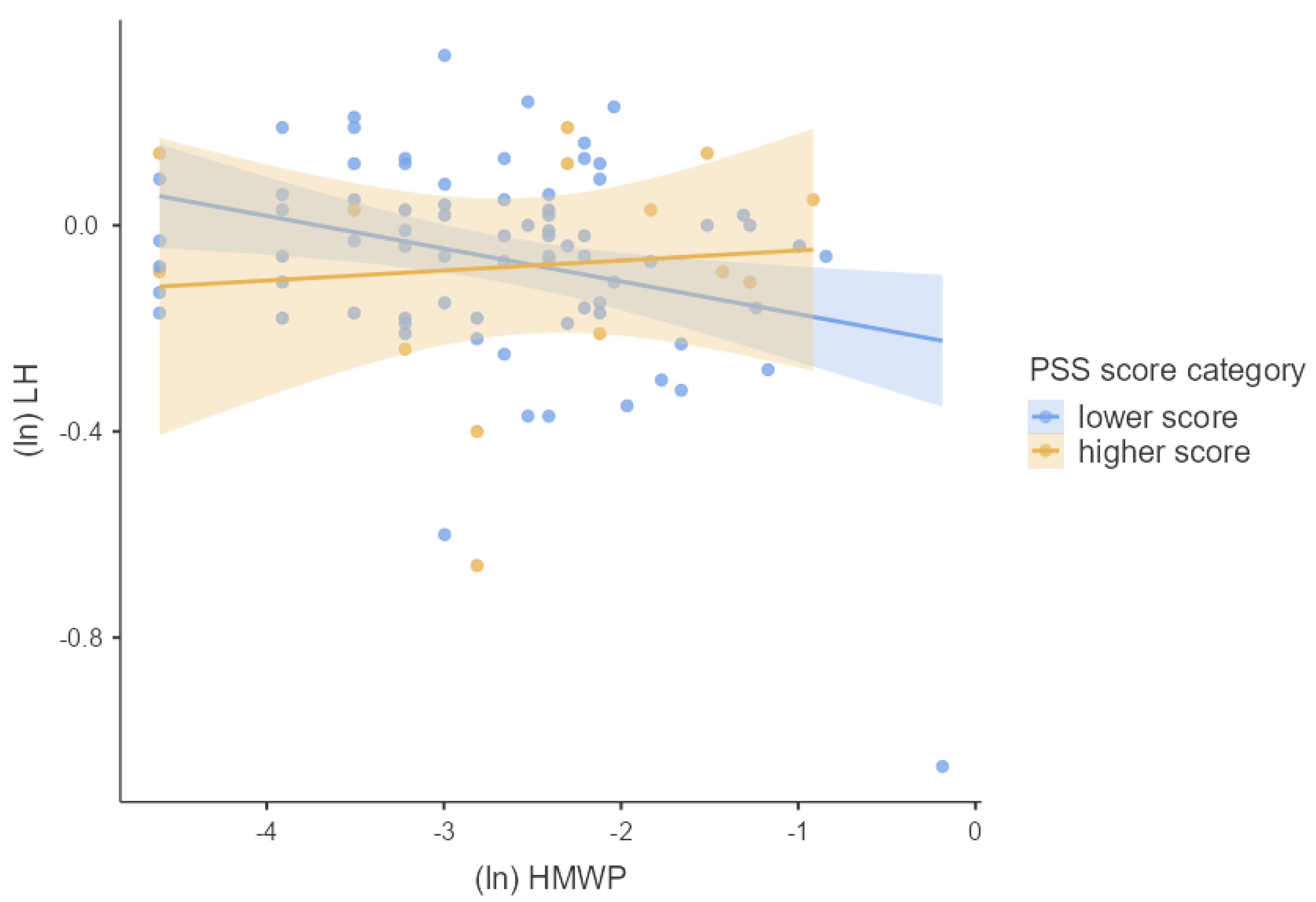
3.4. Associations between Phthalates, Hormones, and Perceived Stress
4. Discussion
4.1. Associations between Phthalate Exposure and Hormonal Concentrations
4.2. Joined Effect of Phthalate Exposure and Perceived Psychosocial Stress on Hormonal Concentrations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gicquel, C.; Le Bouc, Y. Hormonal regulation of fetal growth. Horm. Res. 2006, 65, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S.; Polonsky, K.S.; Larsen, P.R.; Kronenberg, H. Williams Textbook of Endocrinology; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780323297387. [Google Scholar]
- Magon, N.; Kumar, P. Hormones in pregnancy. Niger. Med. J. 2012, 53, 179. [Google Scholar] [CrossRef]
- Villanger, G.D.; Drover, S.S.M.; Nethery, R.C.; Thomsen, C.; Sakhi, A.K.; Øvergaard, K.R.; Zeiner, P.; Hoppin, J.A.; Reichborn-Kjennerud, T.; Aase, H.; et al. Associations between urine phthalate metabolites and thyroid function in pregnant women and the influence of iodine status. Environ. Int. 2020, 137, 105509. [Google Scholar] [CrossRef]
- CDC Phthalates Factsheet. Available online: https://www.cdc.gov/biomonitoring/Phthalates_FactSheet.html (accessed on 6 April 2019).
- Jaakkola, J.J.K.; Knight, T.L. Review The Role of Exposure to Phthalates from Polyvinyl Chloride Products in the Development of Asthma and Allergies: A Systematic Review and Meta-analysis. Environ. Health Perspect. 2008, 116, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef]
- Sathyanarayana, S.; Butts, S.; Wang, C.; Barrett, E.; Nguyen, R.; Schwartz, S.M.; Haaland, W.; Swan, S.H. Early prenatal phthalate exposure, sex steroid hormones, and birth outcomes. J. Clin. Endocrinol. Metab. 2017, 102, 1870–1878. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Kuo, P.; Guo, Y.; Liao, P.; Lee, C. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum. Reprod. 2007, 22, 2715–2722. [Google Scholar] [CrossRef] [Green Version]
- Araki, A.; Mitsui, T.; Miyashita, C.; Nakajima, T.; Naito, H.; Ito, S.; Sasaki, S.; Cho, K.; Ikeno, T.; Nonomura, K.; et al. Association between maternal exposure to di(2-ethylhexyl) phthalate and reproductive hormone levels in fetal blood: The Hokkaido Study on environment and children’s health. PLoS ONE 2014, 9, e109039. [Google Scholar] [CrossRef] [Green Version]
- National Research Council (US) Committee on the Health Risks of Phthalates Phthalate Exposure Assessment in Humans. Available online: https://www.ncbi.nlm.nih.gov/books/NBK215044/ (accessed on 28 April 2019).
- Messerlian, C.; Souter, I.; Gaskins, A.J.; Williams, P.L.; Ford, J.B.; Chiu, Y.-H.; Calafat, A.M.; Hauser, R. Urinary phthalate metabolites and ovarian reserve among women seeking infertility care. Hum. Reprod. 2016, 31, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Mariana, M.; Feiteiro, J.; Verde, I.; Cairrao, E. The effects of phthalates in the cardiovascular and reproductive systems: A review. Environ. Int. 2016, 94, 758–776. [Google Scholar] [CrossRef]
- Ejaredar, M.; Nyanza, E.C.; Ten Eycke, K.; Dewey, D. Phthalate exposure and childrens neurodevelopment: A systematic review. Environ. Res. 2015, 142, 51–60. [Google Scholar] [CrossRef]
- Tsigos, C.; Kyrou, I.; Kassi, E.; Chrousos, G.P. Stress, Endocrine Physiology and Pathophysiology. MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Nestler, E.J.; Hyman, S.E.; Holzman, D.M.; Malenka, R.C. Moecular Neurpharmacology; McGraw-Hill Education: New York, NY, USA, 2015; ISBN 9780071827706. [Google Scholar]
- Joseph, D.N.; Whirledge, S. Stress and the HPA axis: Balancing homeostasis and fertility. Int. J. Mol. Sci. 2017, 18, 2224. [Google Scholar] [CrossRef] [PubMed]
- Ranabir, S.; Reetu, K. Stress and hormones. Indian J. Endocrinol. Metab. 2011, 15, 18. [Google Scholar] [CrossRef]
- Wadhwa, P.D.; Dunkel-Schetter, C.; Chicz-Demet, A.; Porto, M.; Sandman, C.A. Prenatal psychosocial factors and the neuroendocrine axis in human pregnancy. Psychosom. Med. 1996, 58, 432–446. [Google Scholar] [CrossRef] [Green Version]
- Harville, E.W.; Savitz, D.A.; Dole, N.; Herring, A.H.; Thorp, J.M. Stress questionnaires and stress biomarkers during pregnancy. J. Women’s Health 2009, 18, 1425–1433. [Google Scholar] [CrossRef] [Green Version]
- Brunton, P.J. Effects of maternal exposure to social stress during pregnancy: Consequences for mother and offspring. Reproduction 2013, 146, R175–R189. [Google Scholar] [CrossRef] [Green Version]
- Hlisníková, H.; Kolena, B.; Šidlovská, M.; Mlynček, M.; Petrovičová, I. Urinary Phthalate Biomarkers during Pregnancy, and Maternal Endocrine Parameters in Association with Anthropometric Parameters of Newborns. Children 2022, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Kamarck, T.; Mermelstein, R. Perceived Stress Scale (PSS). J. Health Soc. Behav. 1983, 24, 386–396. [Google Scholar] [CrossRef]
- Hlisníková, H.; Petrovičová, I.; Kolena, B.; Šidlovská, M.; Mlynček, M. Effect of prenatal phthalate exposure on the association of maternal hormone levels during early pregnancy and reproductive markers in infants at the age of 3 months. Reprod. Toxicol. 2021, 102, 35–42. [Google Scholar] [CrossRef]
- Koch, H.M.; Rüther, M.; Schütze, A.; Conrad, A.; Pälmke, C.; Apel, P.; Brüning, T.; Kolossa-Gehring, M. Phthalate metabolites in 24-h urine samples of the German Environmental Specimen Bank (ESB) from 1988 to 2015 and a comparison with US NHANES data from 1999 to 2012. Int. J. Hyg. Environ. Health 2017, 220, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Pilka, T.; Petrovicova, I.; Kolena, B.; Zatko, T.; Trnovec, T. Relationship between variation of seasonal temperature and extent of occupational exposure to phthalates. Environ. Sci. Pollut. Res. 2014, 22, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Langer, P.; Kocan, A.; Tajtakova, M.; Petrik, J.; Chovancova, J.; Drobna, B.; Jursa, S.; Pavuk, M.; Trnovec, T.; Seböková, E.; et al. Human thyroid in the population exposed to high environmental pollution by organochlorinated pollutants for several decades. Endocr. Regul. 2005, 39, 13–20. [Google Scholar] [PubMed]
- Aiken, L.S.; West, S.G. Multiple Regression: Testing and Interpreting Interactions; Sage Publications: Newbury Park, CA, USA, 1996; ISBN 978-0761907121. [Google Scholar]
- Schreier, H.M.; Hsu, H.H.; Amarasiriwardena, C.; Coull, B.A.; Schnaas, L.; Téllez-Rojo, M.M.; Tamayo, Y.; Ortiz, M.; Wright, R.J.; Wright, R.O. Mercury and psychosocial stress exposure interact to predict maternal diurnal cortisol during pregnancy. Environ. Health Glob. Access Sci. Source 2015, 14, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbuckle, T.E.; MacPherson, S.; Barrett, E.; Muckle, G.; Séguin, J.R.; Foster, W.G.; Sathyanarayana, S.; Dodds, L.; Fisher, M.; Agarwal, A.; et al. Do stressful life events during pregnancy modify associations between phthalates and anogenital distance in newborns? Environ. Res. 2019, 177, 108593. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.S.; Parlett, L.E.; Sathyanarayana, S.; Redmon, J.B.; Nguyen, R.H.N.; Swan, S.H. Prenatal Stress as a Modifier of Associations between Phthalate Exposure and Reproductive Development: Results from a Multicentre Pregnancy Cohort Study. Paediatr. Perinat. Epidemiol. 2016, 30, 105–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, K.K.; Rosen, E.M.; Barrett, E.S.; Nguyen, R.H.N.; Bush, N.; McElrath, T.F.; Swan, S.H.; Sathyanarayana, S. Joint impact of phthalate exposure and stressful life events in pregnancy on preterm birth. Environ. Int. 2019, 133, 105254. [Google Scholar] [CrossRef] [PubMed]
- Johns, L.E.; Ferguson, K.K.; Soldin, O.P.; Cantonwine, D.E.; Rivera-González, L.O.; Del Toro, A.V.A.; Calafat, A.M.; Ye, X.; Alshawabkeh, A.N.; Cordero, J.F.; et al. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: A longitudinal analysis. Reprod. Biol. Endocrinol. 2015, 13, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Saleh, I. The relationship between urinary phthalate metabolites and polycystic ovary syndrome in women undergoing in vitro fertilization: Nested case-control study. Chemosphere 2022, 286, 131495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yin, W.; Li, P.; Hu, C.; Wang, L.; Li, T.; Gao, E.; Hou, J.; Wang, G.; Wang, X.; et al. Interaction between diet- and exercise-lifestyle and phthalates exposure on sex hormone levels. J. Hazard. Mater. 2019, 369, 290–298. [Google Scholar] [CrossRef]
- Wen, H.-J.; Chen, C.; Wu, M.-T.; Chen, M.-L.; Sun, C.-W.; Wu, W.; Huang, I.; Huang, P.-C.; Yu, T.; Hsiung, C.A.; et al. Phthalate exposure and reproductive hormones and sex-hormone binding globulin before puberty–Phthalate contaminated- foodstuff episode in Taiwan. PLoS ONE 2017, 12, e0175536. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Pan, W.; Shen, X.; Li, C.; Zhou, J.; Liu, J. Urinary levels of phthalate metabolites in women associated with risk of premature ovarian failure and reproductive hormones. Chemosphere 2020, 242, 125206. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.R. Interpretation of reproductive hormones before, during and after the pubertal transition—Identifying health and disordered puberty. Clin. Endocrinol. 2021, 95, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Dekant, W. Grouping of phthalates for risk characterization of human exposures. Toxicol. Lett. 2020, 330, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hlisníková, H.; Petrovičová, I.; Kolena, B.; Šidlovská, M.; Sirotkin, A. Effects and mechanisms of ‘phthalates’ action on reproductive processes and reproductive health: A literature review. Int. J. Environ. Res. Public Health 2020, 17, 6811. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.; Buhrke, T.; Imber, F.; Jessel, S.; Seidel, A.; Völkel, W.; Lampen, A. Agonistic and antagonistic effects of phthalates and their urinary metabolites on the steroid hormone receptors ERα, ERβ, and AR. Toxicol. Lett. 2017, 277, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.; Ji, K. Estrogenic and Androgenic Potential of Phthalates and Their Alternatives. Korean J. Environ. Health Sci. 2016, 42, 169–188. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, S.; Iida, M.; Kobayashi, S.; Jin, K.; Matsuda, T.; Kojima, H. Differential effects of phthalate esters on transcriptional activities via human estrogen receptors α and β, and androgen receptor. Toxicology 2005, 210, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Valsamakis, G.; Papatheodorou, D.C.; Chalarakis, N.; Vrachnis, N.N.; Sidiropoulou, E.J.; Manolikaki, M.; Mantzou, A.; Margeli, A.; Papassotiriou, I.; Chrousos, G.P.; et al. In pregnancy increased maternal STAI trait stress score shows decreased insulin sensitivity and increased stress hormones. Psychoneuroendocrinology 2017, 84, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Braig, S.; Logan, A.; Rothenbacher, D.; Genuneit, J. Psychosocial stress and longitudinally measured gestational weight gain throughout pregnancy: The Ulm SpAtZ Health Study. Sci. Rep. 2020, 10, 1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruessner, J.; Hellhammer, D.; Kirschbaum, C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom. Med. 1999, 61, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, K.E.; Bishop, M.D. Chronic Stress, Cortisol Dysfunction, and Pain: A Psychoneuroendocrine Rationale for Stress Management in Pain Rehabilitation. Phys. Ther. 2014, 94, 1816–1825. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, F.; Zhang, Y.; Wang, W.; Cao, Q. Diminished ovarian reserve induced by chronic unpredictable stress in C57BL/6 mice. Gynecol. Endocrinol. 2020, 36, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Pal, L.; Bevilacqua, K.; Santoro, N.F. Chronic psychosocial stressors are detrimental to ovarian reserve: A study of infertile women. J. Psychosom. Obstet. Gynecol. 2010, 31, 130–139. [Google Scholar] [CrossRef]
- Schliep, K.C.; Mumford, S.L.; Vladutiu, C.J.; Ahrens, K.A.; Perkins, N.J.; Sjaarda, L.A.; Kissell, K.A.; Prasad, A.; Wactawski-Wende, J.; Schisterman, E.F. Perceived stress, reproductive hormones, and ovulatory function. Epidemiology 2015, 26, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Breen, K.M.; Mellon, P.L. Influence of Stress-Induced Intermediates on Gonadotropin Gene Expression in Gonadotrope Cells. Mol. Cell. Endocrinol. 2014, 385, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vierhapper, H.; Waldhausl, W.; Nowotny, P. Gonadotrophin-secretion in adrenocortical insufficiency: Impact of glucocorticoid substitution. Acta Endocrinol. 1982, 101, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Hlisníková, H.; Petrovičová, I.; Kolena, B.; Šidlovská, M.; Sirotkin, A. Effects and mechanisms of ‘phthalates’ action on neurological processes and neural health: A literature review. Pharmacol. Rep. 2021, 73, 386–404. [Google Scholar] [CrossRef] [PubMed]
- Clougherty, J.E.; Shmool, J.L.C.; Kubzansky, L.D. The Role of Non-Chemical Stressors in Mediating Socioeconomic Susceptibility to Environmental Chemicals. Curr. Environ. Health Rep. 2014, 1, 302–313. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.S.; Tucker, P. Critical biological pathways for chronic psychosocial stress and research opportunities to advance the consideration of stress in chemical risk assessment. Am. J. Public Health 2011, 101, 131–139. [Google Scholar] [CrossRef]
- Padula, A.M.; Rivera-Núñez, Z.; Barrett, E.S. Combined Impacts of Prenatal Environmental Exposures and Psychosocial Stress on Offspring Health: Air Pollution and Metals. Curr. Environ. Health Rep. 2020, 7, 89–100. [Google Scholar] [CrossRef]
- Vesterinen, H.M.; Morello-Frosch, R.; Sen, S.; Zeise, L.; Woodruff, T.J. Cumulative effects of prenatal-exposure to exogenous chemicals and psychosocial stress on fetal growth: Systematic-review of the human and animal evidence. PLoS ONE 2017, 12, e0176331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padula, A.M.; Monk, C.; Brennan, P.A.; Borders, A.; Barrett, E.S.; McEvoy, C.T.; Foss, S.; Desai, P.; Alshawabkeh, A.; Wurth, R.; et al. A review of maternal prenatal exposures to environmental chemicals and psychosocial stressors—Implications for research on perinatal outcomes in the ECHO program. J. Perinatol. 2020, 40, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.; Meeker, J.D.; Park, S.; Silva, M.J.; Calafat, A.M. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ. Health Perspect. 2004, 112, 1734–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastiaensen, M.; Malarvannan, G.; Gys, C.; Ait Bamai, Y.; Araki, A.; Covaci, A. Between- and within-individual variability of urinary phthalate and alternative plasticizer metabolites in spot, morning void and 24-h pooled urine samples. Environ. Res. 2020, 191, 110248. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.L.Y.; Lorber, M.; Koch, H.M.; Kolossa-Gehring, M.; Morgan, M.K. Population variability of phthalate metabolites and bisphenol A concentrations in spot urine samples versus 24-or 48-h collections. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 632–640. [Google Scholar] [CrossRef] [PubMed]


| Mean (±SD) | % (n) | ||
|---|---|---|---|
| Age | 30.80 ± 4.97 | ||
| BMI (nmiss = 2) | 23.05 ± 4.07 | ||
| Week of pregnancy | 10.46 ± 1.80 | ||
| PSS score | 15.20 ± 4.82 | ||
| PSS score category | Lower score (≤19 points) | 83.30% (n = 75) | |
| Higher score (≥20 points) | 16.70% (n = 15) | ||
| Education (nmiss = 1) | High school | 38.20% (n = 34) | |
| College/university | 61.80% (n = 55) | ||
| Living area | Rural | 54.40% (n = 48) | |
| Urban | 45.60% (n = 40) | ||
| Number of previous pregnancies | 0 | 50.00% (n = 45) | |
| 1 | 28.90% (n = 26) | ||
| 2 | 14.40% (n = 13) | ||
| ≥3 | 6.70% (n = 6) | ||
| Active smoking | Smoker | 5.60% (n = 5) | |
| Former smoker | 28.90% (n = 26) | ||
| Non-smoker | 65.60% (n = 59) | ||
| Passive smoking (nmiss = 1) | Yes | 20.22% (n = 18) | |
| No | 79.78% (n = 71) |
| Compound Name | % ≥LOQ | MIN | MED | MAX | MEAN | SD |
|---|---|---|---|---|---|---|
| MMP | 30.68 | ≤LOQ | ≤LOQ | 26.21 | 3.01 | 4.09 |
| MEP | 94.32 | ≤LOQ | 20.90 | 629.28 | 55.78 | 95.12 |
| MiBP | 92.05 | ≤LOQ | 14.04 | 420.07 | 24.85 | 47.88 |
| MnBP | 90.91 | ≤LOQ | 17.17 | 415.45 | 27.40 | 48.09 |
| OH-MiBP | 77.27 | ≤LOQ | 1.89 | 38.61 | 3.06 | 4.61 |
| OH-MnBP | 94.32 | ≤LOQ | 6.94 | 132.97 | 10.34 | 16.19 |
| MnPeP | 0.00 | ≤LOQ | ≤LOQ | ≤LOQ | ≤LOQ | 0.00 |
| MCHP | 0.00 | ≤LOQ | ≤LOQ | ≤LOQ | ≤LOQ | 0.00 |
| MBzP | 52.27 | ≤LOQ | 1.17 | 28.72 | 1.84 | 3.43 |
| OH-MEHP | 86.36 | ≤LOQ | 4.02 | 43.20 | 6.09 | 6.74 |
| oxo-MEHP | 85.23 | ≤LOQ | 3.30 | 25.73 | 4.95 | 4.99 |
| cx-MEPP | 96.59 | ≤LOQ | 5.74 | 53.07 | 8.60 | 8.69 |
| MiNP | 0.00 | ≤LOQ | ≤LOQ | ≤LOQ | ≤LOQ | 0.00 |
| OH-MiNP | 82.95 | ≤LOQ | 4.16 | 82.25 | 8.32 | 12.39 |
| oxo-MiNP | 29.55 | ≤LOQ | ≤LOQ | 63.41 | 1.96 | 6.70 |
| cx-MiNP | 78.41 | ≤LOQ | 2.03 | 151.04 | 4.60 | 16.10 |
| MnOP | 0.00 | ≤LOQ | ≤LOQ | ≤LOQ | ≤LOQ | 0.00 |
| ΣDiBP | - | 0.01 | 0.09 | 2.45 | 0.16 | 0.28 |
| ΣDnBP | - | 0.01 | 0.11 | 2.03 | 0.17 | 0.25 |
| ΣDEHP | - | 0.01 | 0.05 | 0.41 | 0.07 | 0.07 |
| ΣDiNP | - | 0.01 | 0.03 | 0.94 | 0.05 | 0.10 |
| ΣLMWP | - | 0.04 | 0.41 | 3.40 | 0.63 | 0.68 |
| ΣHMWP | - | 0.01 | 0.08 | 1.04 | 0.11 | 0.13 |
| Compound Name | Units | MIN | MED | MAX | MEAN | SD |
|---|---|---|---|---|---|---|
| FSH | mIU/mL | 0.00 | 0.01 | 0.37 | 0.04 | 0.06 |
| LH | mIU/mL | 0.09 | 0.93 | 2.12 | 0.94 | 0.36 |
| Estradiol | pg/mL | 851.00 | 3079.00 | 20,800.00 | 4007.12 | 3280.26 |
| Testosterone | ng/mL | 0.16 | 0.68 | 1.51 | 0.74 | 0.29 |
| TSH | μIU/mL | 0.03 | 1.44 | 3.90 | 1.66 | 0.94 |
| FT3 | pg/mL | 0.99 | 3.30 | 10.40 | 5.25 | 1.03 |
| FT4 | ng/mL | 0.08 | 0.82 | 3.82 | 1.68 | 0.54 |
| Cortisol | μg/mL | 0.85 | 17.53 | 198.00 | 84.99 | 25.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlisníková, H.; Nagyová, M.; Kolena, B.; Mlynček, M.; Trnovec, T.; Petrovičová, I. The Joint Effect of Perceived Psychosocial Stress and Phthalate Exposure on Hormonal Concentrations during the Early Stage of Pregnancy: A Cross-Sectional Study. Children 2022, 9, 1561. https://doi.org/10.3390/children9101561
Hlisníková H, Nagyová M, Kolena B, Mlynček M, Trnovec T, Petrovičová I. The Joint Effect of Perceived Psychosocial Stress and Phthalate Exposure on Hormonal Concentrations during the Early Stage of Pregnancy: A Cross-Sectional Study. Children. 2022; 9(10):1561. https://doi.org/10.3390/children9101561
Chicago/Turabian StyleHlisníková, Henrieta, Miroslava Nagyová, Branislav Kolena, Miloš Mlynček, Tomáš Trnovec, and Ida Petrovičová. 2022. "The Joint Effect of Perceived Psychosocial Stress and Phthalate Exposure on Hormonal Concentrations during the Early Stage of Pregnancy: A Cross-Sectional Study" Children 9, no. 10: 1561. https://doi.org/10.3390/children9101561






