Birth Weight and Body Composition as Determined by Isotopic Dilution with Deuterium Oxide in 6- to 8-Year-Old South African Children †
Abstract
1. Introduction
2. Materials and Methods
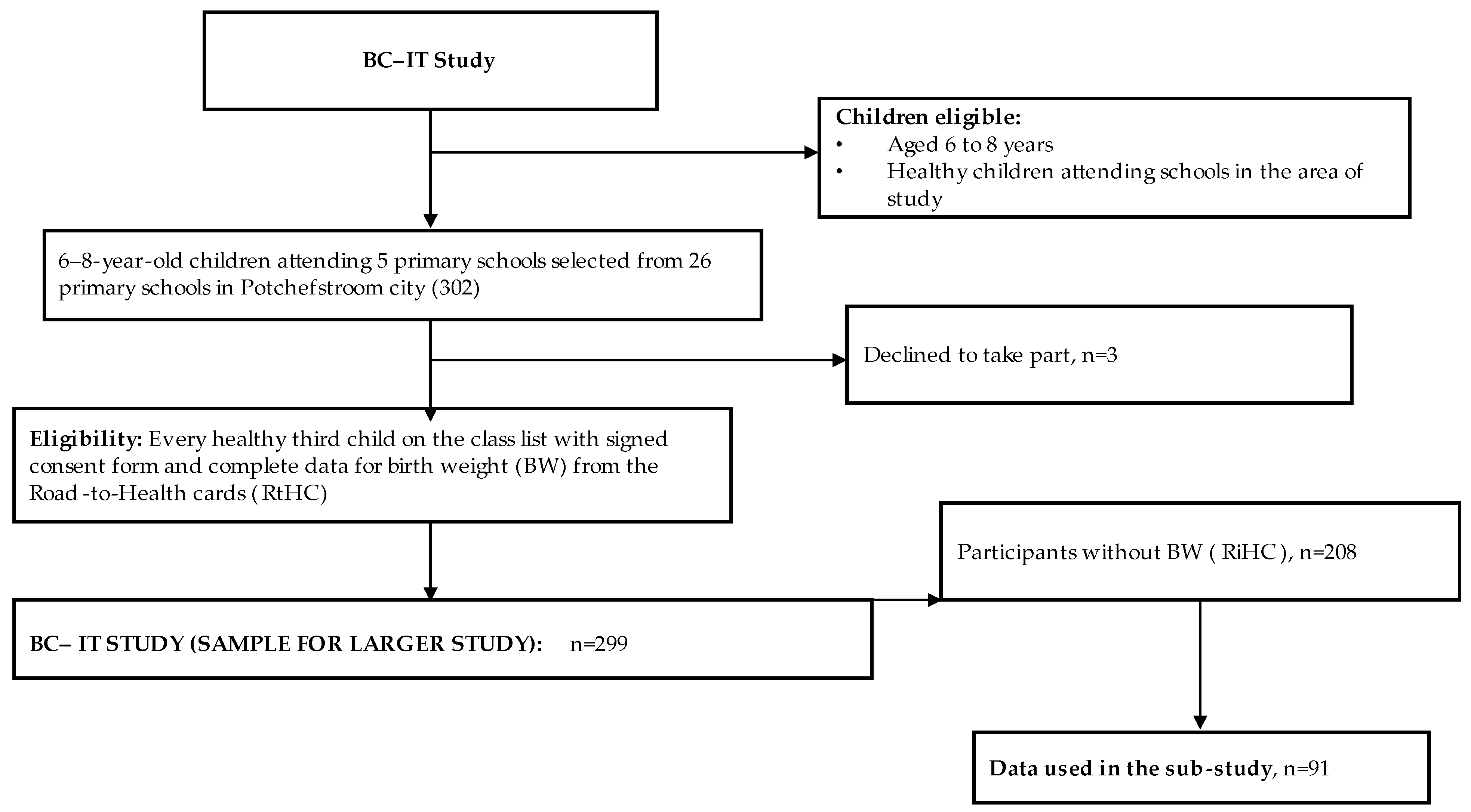
2.1. Study Design and Participants
2.2. Measuring Instruments
2.2.1. Socio-Demographic Questionnaire
2.2.2. Birth Weight
2.2.3. Anthropometric Measurements
2.2.4. Body Composition by Isotopic Dilution with Deuterium (D2O) Oxide
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Cesare, M.; Sorić, M.; Bovet, P.; Miranda, J.J.; Bhutta, Z.; Stevens, G.A.; Laxmaiah, A.; Kengne, A.; Bentham, J. The epidemiological burden of obesity in childhood: A worldwide epidemic requiring urgent action. BMC Med. 2019, 17, 212–232. [Google Scholar] [CrossRef] [PubMed]
- NCD Child. Children and Non-Communicable Disease. Global Burden Report. 2019. Available online: https://www.ncdchild.org/wp-content/uploads/2021/03/ncdchild_global_burden-report-2019.pdf (accessed on 6 July 2020).
- Masocha, V.; Monyeki, M.A.; Czy, S.H. Longitudinal relationships between changes in body composition and changes in selected metabolic risk factors (abdominal obesity and blood pressure) among South African adolescents. Peer J. 2020, 8, e9331. [Google Scholar] [CrossRef]
- Larqué, E.; Labayen, I.; Flodmark, C.; Lissau, I.; Czernin, S.; Moreno, L.A.; Pietrobelli, A.; Widhalm, K. From conception to infancy—Early risk factors for childhood obesity. Nat. Rev. Endocrinol. 2019, 15, 456–478. [Google Scholar] [CrossRef] [PubMed]
- Kumordzie, S.K.; Okronipa, H.; Arimond, M.; Adu-Afarwuah, S.; Ocansey, M.E.; Young, R.R.; Bentil, H.J.; Tamakloe, S.M.; Oaks, B.; Dewey, K.G. Maternal and child factors associated with child body fatness in a Ghanaian cohort. Public Health Nutr. 2019, 23, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Rito, A.I.; Buoncristiano, M.; Spinelli, A.; Salanave, B.; Kunešová, M.; Hejgaard, T.; Solano, M.G.; Fijalkowska, A.; Sturua, L.; Hyska, J.; et al. Association between characteristics at birth, breastfeeding and obesity in 22 countries: The WHO European childhood obesity surveillance initiative—COSI 2015/2017. Obes. Facts 2019, 12, 226–243. [Google Scholar] [CrossRef]
- Campbell, M.K. Biological, environmental, and social influences on childhood obesity. Pediatr. Res. 2016, 79, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Ayina, C.N.A.; Assomo, N.P.B.; Jerson, M.N.; Bilog, N.C.; Ahmadou; Bindi, N.J.G.; Etaga, N.B.; Temfemo, A.; Mbanya, J.C.; Sobngwi, E.; et al. Obesity and risk of comorbidity: Prevalence and associated factors in children aged 6 to 9 years in public and private schools in Douala-Cameroon. Cent. Afr. J. Public Health 2020, 6, 192–199. [Google Scholar] [CrossRef]
- Wilcox, A.J. On the importance and unimportance of birthweight. Int. J. Epidemiol. 2001, 30, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Kaul, P.; Bowker, S.L.; Savu, A.; Yeung, R.O.; Donovan, L.E.; Ryan, E.A. Association between maternal diabetes, being large for gestational age and breast-feeding on being overweight or obese in childhood. Diabetologia 2018, 62, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Yeung, S.L.A.; Kwok, M.; Leung, J.Y.Y.; Lin, S.L.; Hui, L.L.; Leung, G.M.; Schooling, C.M. Birth weight, gestational age and late adolescent liver function using twin status as instrumental variable in a Hong Kong Chinese birth cohort: Children of 1997. Prev. Med. 2018, 111, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Risnes, K.R.; Vatten, L.J.; Baker, J.L.; Jameson, K.; Sovio, U.; Kajantie, E.; Osler, M.; Morley, R.; Jokela, M.; Painter, R.C.; et al. Birthweight and mortality in adulthood: A systematic review and meta-analysis. Int. J. Epidemiol. 2011, 40, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Huang, T.; Li, Y.; Zheng, Y.; Manson, J.E.; Hu, F.B.; Qi, L. Low birthweight and risk of type 2 diabetes: A Mendelian randomisation study. Diabetologia 2016, 59, 1920–1927. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, D.; Tikkanen, E.; Gustafsson, S.; Priest, J..; Burgess, S.; Ingelsson, E. Birthweight, Type 2 Diabetes Mellitus, and cardiovascular disease: Addressing the barker hypothesis with mendelian randomization. Circ. Genom. Precis. Med. 2018, 11, e002054. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Yang, Z.; Yang, Z.; Wang, X.; Gao, D.; Dong, Y.; Ma, J.; Ma, Y. Association of high birthweight with overweight and obesity in Chinese students aged 6–18 years: A national, cross-sectional study in China. BMJ Open 2019, 9, e024532. [Google Scholar] [CrossRef]
- Melaku, Y.A.; Gill, T.K.; Taylor, A.W.; Appleton, S.L.; Gonzalez-Chica, D.; Adams, R.; Achoki, T.; Shi, Z.; Renzaho, A. Trends of mortality attributed to child and maternal undernutrition, overweight/obesity and dietary risk factors of non-communicable diseases in sub-Saharan Africa, 1990-2015: Findings from Global Burden of Disease Study 2015. Public Health Nutr. 2018, 22, 827–840. [Google Scholar] [CrossRef]
- Wilkes, M.; Thornton, J.; Horlick, M.; Sopher, A.; Wang, J.; Widen, E.M.; Pierson, R.; Gallagher, D. Relationship of BMI z score to fat percent and fat mass in multiethnic prepubertal children. Pediatr. Obes. 2019, 14, e12463. [Google Scholar] [CrossRef]
- Adom, T.; Kengne, A.P.; De Villiers, A.; Boatin, R.; Puoane, T. Diagnostic accuracy of body mass index in defining childhood obesity: Analysis of cross-sectional data from Ghanaian children. Int. J. Environ. Res. Public Health 2020, 17, 36. [Google Scholar] [CrossRef]
- Nerud, K.; Smith, A.; Nielsen, R.; Neely, D. The combined effect of infant birthweight and maternal determinants of health on the development of childhood obesity: A systematic review. J. Obes. Chronic Dis. 2018, 2, 1–7. [Google Scholar] [CrossRef]
- Bernhardsen, G.P.; Stensrud, T.; Nystad, W.; Dalene, K.E.; Kolle, E.; Ekelund, U. Early life risk factors for childhood obesity—Does physical activity modify the associations? The MoBa cohort study. Scand. J. Med. Sci. Sports 2019, 29, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
- Pruszkowska-Przybylska, P.; Sitek, A.; Rosset, I.; Sobalska-Kwapis, M.; Słomka, M.; Strapagie, D.; Żądzińska, E. The association between socioeconomic status, duration of breastfeeding, parental age and birth parameters with BMI, body fat and muscle mass among prepubertal children in Poland. Anthr. Anz. 2019, 76, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Freire, J.A.; Lemos, J.O.; de Sousa, A.F.; Meneses, C.C.; Rondó, P.H.C. Association between weight at birth and body composition in childhood: A Brazilian cohort study. Early Hum. Dev. 2015, 91, 445–449. [Google Scholar] [CrossRef]
- Moeng-Mahlangu, L.; Monyeki, M.A.; Reilly, J.J.; Mchiza, Z.J.; Moleah, T.; Loechl, C.U.; Kruger, H.S. The level of agreement between objectively determined body composition versus perceived body image outcomes in 6 to 8 year old South African children: BC—IT study. PLoS ONE 2020, 15, e0237399. [Google Scholar] [CrossRef]
- Sedumedi, C.M.; Janssen, X.; Reilly, J.J.; Kruger, H.S.; Monyeki, M.A. Association between Objectively Determined Physical Activity Levels and Body Composition in 6–8-Year-Old Children from a Black South African Population: BC–IT Study. Int. J. Environ. Res. Public Health 2021, 18, 6453. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, H.; Avenant, T.; Goga, A. Completeness of the Road-to-Health Booklet and Road-to-Health Card: Results of cross-sectional surveillance at a provincial tertiary hospital. South. Afr. J. HIV Med. 2018, 19, 765. [Google Scholar] [CrossRef]
- Tarwa, C.; De Villiers, F.P.R. The use of the road to health card in monitoring child health. South Afr. Fam. Pract. 2007, 49, 15. [Google Scholar] [CrossRef]
- World Health Organization. Global Nutrition Targets 2025: Low Birth Weight Policy Brief; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Norwegian Institute of Public Health. Birth Weight in Norway, Fsact Sheet: Norwegian Institute of Public Health. 2015. Available online: https://www.fhi.no/hn/statistikk/statistikk3/fodselsvekt-i-norge-faktaark/ (accessed on 20 April 2020).
- Evensen, E.; Emaus, N.; Kokkvoll, A.; Wilsgaard, T.; Furberg, A.S.; Skeie, G. The relation between birthweight, childhood body mass index, and overweight and obesity in late adolescence: A longitudinal cohort study from Norway, The Tromsø Study, Fit Futures. BMJ Open 2017, 7, e015576. [Google Scholar] [CrossRef]
- Stewart, A.; Marfell-Jones, M.; Olds, T.; De Ridder, H. International Standards for Anthropometric Assessment; ISAK: Lower Hutt, New Zealand, 2011. [Google Scholar]
- De Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Introduction to Body Composition Assessment Using the Deuterium Dilution Technique with Analysis of Saliva Samples by Fourier Transform Infrared Spectrometry; Human Health Series No. 12; IAEA: Vienna, Austria, 2011. [Google Scholar]
- El Hamdouchi, A.; Adom, T.; Aouidet, A.; Agueh, V.D.; Diouf, A.; Joonus, N.I.; Leyna, G.H.; Mbithe, K.D.; Moleah, T.; Monyeki, M.A.; et al. Protocol for validating simple measures of body fatness and physical activity of children in twelve African countries: The round-it Africa Study. Afr. J. Phys. Act. Health Sci. 2019, 25, 142–173. [Google Scholar]
- Owino, V.O.; Slater, C.; Loechl, C.U. Using stable isotope techniques in nutrition assessments and tracking of global targets post-2015. Proc. Nutr. Soc. 2017, 76, 495–503. [Google Scholar] [CrossRef]
- McCarthy, H.D.; Cole, T.J.; Fry, T.; Jebb, S.A.; Prentice, A.M. Body fat reference curves for children. Int. J. Obes. 2006, 30, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Chomtho, S.; Wells, J.C.; Williams, J.E.; Lucas, A.; Fewtrell, M.S. Associations between birth weight and later body composition: Evidence from the 4-component model. Am. J. Clin. Nutr. 2008, 88, 1040–1048. [Google Scholar] [CrossRef]
- Moura-dos Santos, M.A.; Verçosa, M.F.; Gomes, T.N.Q.F.; Maia, J.A.R.; Leandro, C.G. Birth weight, physical growth and body composition in children: A longitudinal study. Rev. Nutr. 2018, 31, 287–297. [Google Scholar] [CrossRef]
- Choukem, S.; Tochie, J.N.; Sibetcheu, A.T.; Nansseu, J.R.; Hamilton-Shield, J.P. Overweight/obesity and associated cardiovascular risk factors in sub-Saharan African children and adolescents: A scoping review. Int. J. Pediatr. Endocrinol. 2020, 2020, 6. [Google Scholar] [CrossRef]
- Patro, B.; Liber, A.; Zalewski, B.; Poston, L.; Szajewska, H.; Koletzko, B. Maternal and paternal body mass index and offspring obesity: A systematic review. Ann. Nutr. Metab. 2013, 63, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, J.; Richmond, R.C.; Palmer, T.M.; Feenstra, B.; Rangarajan, J.; Metrustry, S.; Cavadino, A.; Paternoster, L.; Armstrong, L.L.; De Silva, N.M.; et al. Genetic evidence for causal relationships between maternal obesity-related traits and birth weight. JAMA 2016, 315, 1129–1140. [Google Scholar] [CrossRef]
- Navti, L.K.; Ferrari, U.; Tange, E.; Pozza, S.B.; Parhofer, K.G. Contribution of socioeconomic status, stature and birth weight to obesity in Sub-Saharan Africa: Cross-sectional data from primary school-age children in Cameroon. BMC Public Health 2014, 14, 320. [Google Scholar] [CrossRef]
- Hou, W.W.; Tse, M.A.; Lam, T.H.; Leung, G.M.; Schooling, C.M. Adolescent testosterone, muscle mass and glucose metabolism: Evidence from the ’Children of 1997’ birth cohort in Hong Kong. Diabet. Med. 2015, 32, 505–512. [Google Scholar] [CrossRef]
- Yeung, C.H.C.; Au Yeung, S.L.; Fong, S.S.M.; Schooling, C.M. Lean mass, grip strength and risk of type 2 diabetes: A bi-directional Mendelian randomisation study. Diabetologia 2019, 62, 789–799. [Google Scholar] [CrossRef]
- Catalano, P.M.; Drago, N.M.; Amini, S.B. Factors affecting fetal growth and body composition. Am. J. Obstet. Gynecol. 1995, 172, 1459–1463. [Google Scholar] [CrossRef]
- Williams, D.P.; Going, S.B.; Lohman, T.G.; Harsha, D.W.; Srinivasan, S.R.; Webber, L.S.; Berenson, G.S. Body fatness and risk for elevated blood pressure, total cholesterol, and serum lipoprotein ratios in children and adolescents. Am. J. Public Health 1992, 82, 358–363. [Google Scholar] [CrossRef]
- Tshotetsi, L.; Dzikiti, L.; Hajison, P.; Feresu, S. Maternal factors contributing to low birth weight deliveries in Tshwane District, South Africa. PLoS ONE 2019, 14, e0213058. [Google Scholar] [CrossRef]
- Tessema, Z.T.; Tamirat, K.S.; Teshale, A.B.; Tesema, G.A. Prevalence of low birth weight and its associated factor at birth in Sub-Saharan Africa: A generalized linear mixed model. PLoS ONE 2021, 16, e0248417. [Google Scholar] [CrossRef]
- Yajnik, C.S.; Fall, C.H.; Coyaji, K.J.; Hirve, S.S.; Rao, S.; Barker, D.J.; Joglekar, C.; Kellingray, S. Neonatal anthropometry: The thin-fat Indian baby. The Pune Maternal Nutrition Study. Int. J. Obes. 2003, 27, 173–180. [Google Scholar] [CrossRef]
| Variables | N | Minimum | Maximum | Mean | SD |
|---|---|---|---|---|---|
| Age (Years) | 91 | 6.0 | 8.8 | 7.7 | 0.7 |
| BW (g) | 91 | 1000 | 4320 | 3053.9 | 538.2 |
| Weight (kg) | 91 | 15.1 | 48.7 | 25.0 | 5.9 |
| Height (cm) | 91 | 103.5 | 138.4 | 122.2 | 7.0 |
| FFM (kg) | 91 | 10.7 | 25.9 | 17.7 | 3.2 |
| FM (kg) | 91 | 2.7 | 22.8 | 7.3 | 3.5 |
| FM (%) | 91 | 12.0 | 46.8 | 28.1 | 7.2 |
| TBW (ℓ; D2O) | 91 | 8.3 | 19.9 | 13.7 | 2.4 |
| BMI for age Z-score | 91 | −2.4 | 3.6 | 0.3 | 1.2 |
| Total Group (n = 91) | Boys (n = 40) | Girls (n = 51) | ||
|---|---|---|---|---|
| Birth weight categories | N (%) | N (%) | N (%) | χ2 test for sex difference |
| LBW | 12 (13) | 5 (12) | 7 (14) | |
| NBW | 78 (87) | 35 (88) | 44 (86) | 0.864 |
| D2O fat percentage categories | ||||
| Underweight | 4 (4) | 2 (5) | 2 (3.9) | |
| Normal weight | 53 (58) | 30 (75) | 23 (45.1) | 0.016 |
| Overweight | 19 (21) | 6 (15) | 13 (25.5) | |
| Obese | 15 (17) | 2 (5) | 13 (25.5) | |
| BMI z-score categories | ||||
| Grades 1 and 2 thinness | 7 (8) | 3 (7) | 4 (8) | |
| Normal | 67 (74) | 33 (83) | 34 (67) | 0.58 |
| Overweight | 12 (13) | 4 (10) | 8 (15) | |
| Obese | 5 (5) | 0 (0) | 5 (10) |
| LBW Categories (n = 12) | NBW Group (n = 79) | p-Value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (years) | 7.45 ± 0.9 | 7.71 ± 0.7 | 0.38 |
| Birth weight (kg) | 2.10 ± 0.40 | 3.20 ± 0.38 | <0.0001 * |
| Weight (kg) | 22.27 ± 6.5 | 25.48 ± 5.8 | 0.1 |
| Height (cm) | 117.42 ± 10.6 | 122.97 ± 6.1 | 0.01 |
| FFM (kg; D2O) | 16.47 ± 4.3 | 17.95 ± 3.0 | 0.27 |
| FM (kg; D2O) | 5.81 ± 2.8 | 7.53 ± 3.6 | 0.08 |
| FM (%; D2O) | 25.59 ± 6.22 | 28.51 ± 7.27 | 0.16 |
| BW Unadjusted | BW Adjusted for Age and Sex | BW Adjusted for Age, Sex, and SES | ||
|---|---|---|---|---|
| Weight | r | 0.23 | 0.20 | 0.21 |
| p | 0.03 * | 0.06 | 0.20 | |
| Height | r | 0.33 | 0.31 | 0.22 |
| p | <0.001 ** | 0.003 | 0.16 | |
| FFM (kg; D2O) | r | 0.27 | 0.21 | 0.27 |
| p | 0.01 * | 0.51 | 0.10 | |
| FM (%; D2O) | r | 0.11 | 0.16 | 0.09 |
| p | 0.30 | 0.12 | 0.57 | |
| FM (kg; D2O) | r | 0.23 | 0.16 | 0.19 |
| p | 0.03 * | 0.14 | 0.24 |
| Dependent Variable | Unstandardised β | Adjusted r Square | p Value | 95% CI | |
|---|---|---|---|---|---|
| Crude models | |||||
| Total group | FFM (kg; D2O) | 0.24 | 0.057 | 0.01 | 0.032; 0.441 |
| FM (kg; D2O) | 0.21 | 0.044 | 0.02 | 0.001; 0.412 | |
| FM (%; D2O) | 0.18 | 0.033 | 0.30 | −0.027; 0.338 | |
| Boys | FFM (kg; D2O) | 0.13 | 0.430 | 0.43 | −0.198; 0.454 |
| FM (kg; D2O) | 0.24 | 0.057 | 0.08 | −0.081; 0.557 | |
| FM (%;D2O) | 0.25 | 0.063 | 0.12 | −0.067; 0.568 | |
| Girls | FFM (kg; D2O) | 0.32 | 0.085 | 0.02 | 0.050; 0.574 |
| FM (kg; D2O) | 0.18 | 0.202 | 0.06 | −0.101; 0.464 | |
| FM (%;D2O) | 0.13 | 0016 | 0.38 | −0.159; 0.411 | |
| Adjusted models * | |||||
| Total group | FFM (kg; D2O) | 0.18 | 0.390 | 0.07 | −0.013; 0.381 |
| FM (kg; D2O) | 0.17 | 0.110 | 0.04 | 0.031; 0.375 | |
| FM (%; D2O) | 0.17 | 0.075 | 0.17 | -0.069; 0.405 | |
| Boys | FFM (kg; D2O) | 0.24 | 0.320 | 0.16 | −0.102; 0.576 |
| FM (kg; D2O) | 0.27 | 0.160 | 0.15 | −0.108; 0.653 | |
| FM (%; D2O) | 0.22 | 0.094 | 0.26 | −0.171; 0.607 | |
| Girls | FFM (kg; D2O) | 0.12 | 0.510 | 0.36 | −0.124; 0.381 |
| FM (kg; D2O) | 0.12 | 0.100 | 0.37 | −0.158;0.408 | |
| FM (%; D2O) | 0.15 | 0.050 | 0.37 | −0.182; 0.477 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monyeki, M.A.; Sedumedi, C.M.; Reilly, J.J.; Janssen, X.; Kruger, H.S.; Kruger, R.; Loechl, C.U. Birth Weight and Body Composition as Determined by Isotopic Dilution with Deuterium Oxide in 6- to 8-Year-Old South African Children. Children 2022, 9, 1597. https://doi.org/10.3390/children9101597
Monyeki MA, Sedumedi CM, Reilly JJ, Janssen X, Kruger HS, Kruger R, Loechl CU. Birth Weight and Body Composition as Determined by Isotopic Dilution with Deuterium Oxide in 6- to 8-Year-Old South African Children. Children. 2022; 9(10):1597. https://doi.org/10.3390/children9101597
Chicago/Turabian StyleMonyeki, Makama Andries, Caroline Molete Sedumedi, John J. Reilly, Xanne Janssen, Herculina Salome Kruger, Ruan Kruger, and Cornelia U. Loechl. 2022. "Birth Weight and Body Composition as Determined by Isotopic Dilution with Deuterium Oxide in 6- to 8-Year-Old South African Children" Children 9, no. 10: 1597. https://doi.org/10.3390/children9101597
APA StyleMonyeki, M. A., Sedumedi, C. M., Reilly, J. J., Janssen, X., Kruger, H. S., Kruger, R., & Loechl, C. U. (2022). Birth Weight and Body Composition as Determined by Isotopic Dilution with Deuterium Oxide in 6- to 8-Year-Old South African Children. Children, 9(10), 1597. https://doi.org/10.3390/children9101597







