Increased Risk of Tourette Syndrome with Leukotriene Modifier Use in Children with Allergic Diseases and Asthma: A Nationwide Population-Based Study
Abstract
1. Introduction
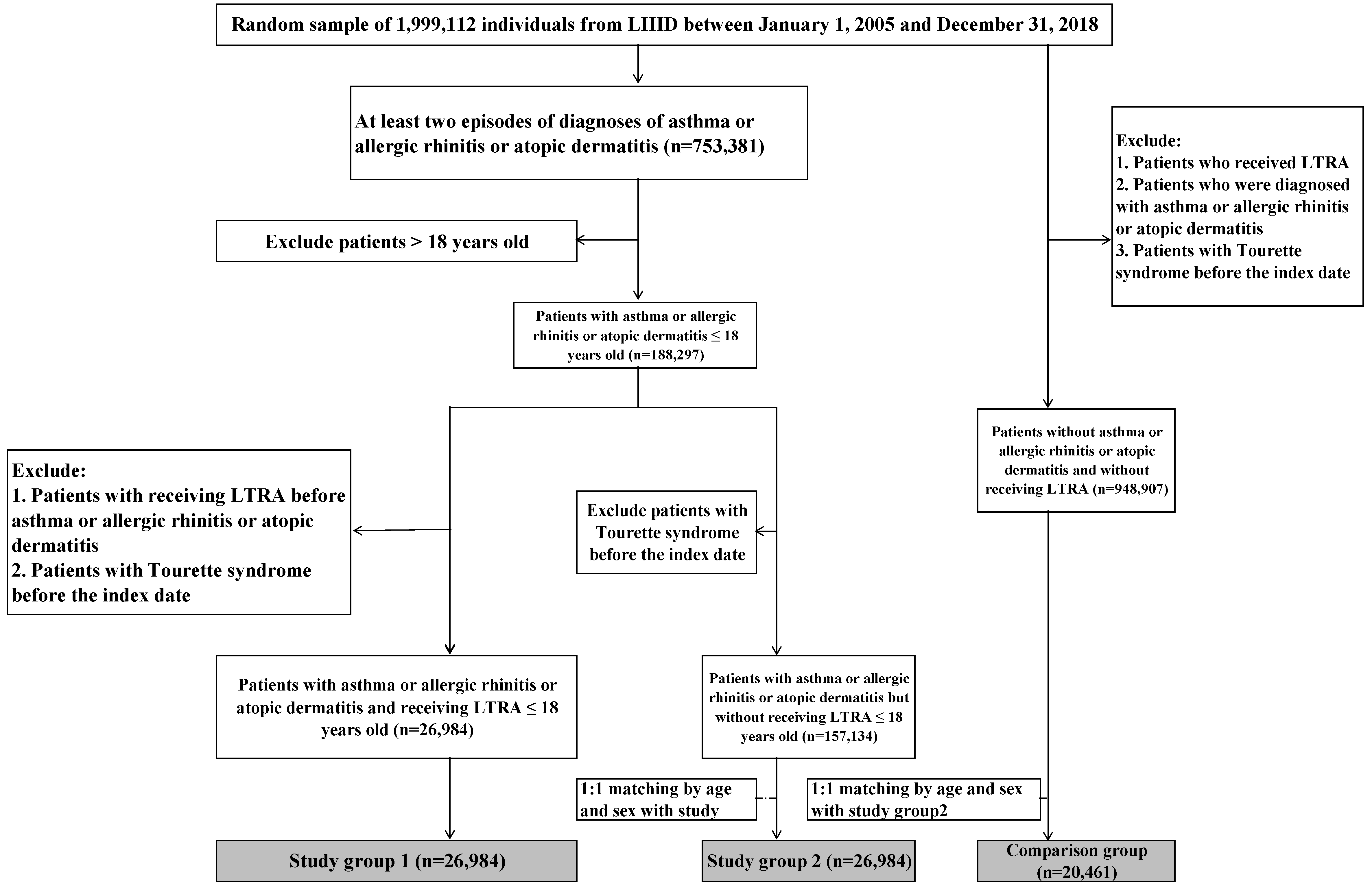
2. Materials and Methods
2.1. Study Population
2.2. Definition
2.3. Confounders
2.4. Outcome Measurement
2.5. Statistical Analysis
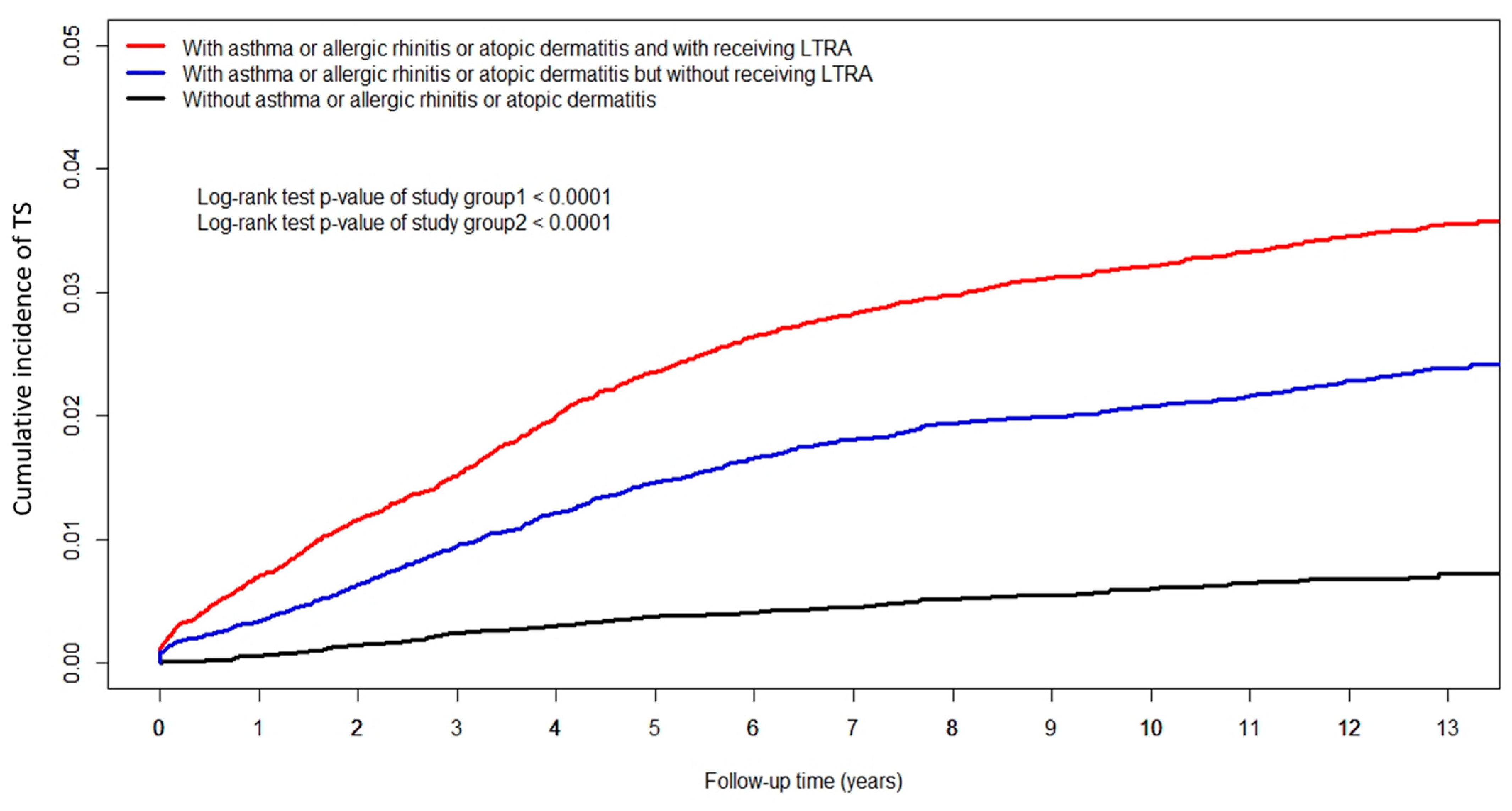
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Leckman, J.F.; Peterson, B.S.; Pauls, D.L.; Cohen, D.J. Tic disorders. Psychiatr. Clin. N. Am. 1997, 20, 839–861. [Google Scholar] [CrossRef]
- Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013.
- Wang, H.S.; Kuo, M.F. Tourette’s syndrome in Taiwan: An epidemiological study of tic disorders in an elementary school at Taipei County. Brain Dev. 2003, 25 (Suppl. 1), S29–S31. [Google Scholar] [CrossRef]
- Robertson, M.M. The prevalence and epidemiology of Gilles de la Tourette syndrome. Part 1: The epidemiological and prevalence studies. J. Psychosom. Res. 2008, 65, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Li, Y.F.; Muo, C.H.; Chen, S.C.; Chin, Z.N.; Kuo, H.T.; Lin, H.C.; Sung, F.C.; Tsai, C.H.; Chou, I.C. Correlation of Tourette syndrome and allergic disease: Nationwide population-based case-control study. J. Dev. Behav. Pediatr. 2011, 32, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.S.; Shen, E.Y.; Shyur, S.D.; Chiu, N.C. Association of allergy with Tourette’s syndrome. J. Formo. Med. Assoc. 1999, 98, 492–495. [Google Scholar]
- Finegold, I. Allergy and Tourette’s syndrome. Ann. Allergy 1985, 55, 119–121. [Google Scholar]
- Kim, H.; Moote, W.; Mazza, J. Tourette’s syndrome in patients referred for allergy evaluation. Ann. Allergy Asthma Immunol. 1997, 79, 347–349. [Google Scholar] [CrossRef]
- Scott, J.P.; Peters-Golden, M. Antileukotriene agents for the treatment of lung disease. Am. J. Respir. Crit. Care Med. 2013, 188, 538–544. [Google Scholar] [CrossRef]
- Dahlén, S.E.; Dahlén, B.; Drazen, J.M. Asthma treatment guidelines meet the real world. N. Engl. J. Med. 2011, 364, 1769–1770. [Google Scholar] [CrossRef]
- Peters-Golden, M.; Henderson, W.R., Jr. The role of leukotrienes in allergic rhinitis. Ann. Allergy Asthma Immunol. 2005, 94, 609–618. [Google Scholar] [CrossRef]
- Diamant, Z.; Mantzouranis, E.; Bjermer, L. Montelukast in the treatment of asthma and beyond. Expert. Rev. Clin. Immunol. 2009, 5, 639–658. [Google Scholar] [CrossRef] [PubMed]
- Knorr, B.; Franchi, L.M.; Bisgaard, H.; Vermeulen, J.H.; LeSouef, P.; Santanello, N.; Michele, T.M.; Reiss, T.F.; Nguyen, H.H.; Bratton, D.L. Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years. Pediatrics 2001, 108, e48. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.; Leung, T.F.; Leung, A.K. Clinical effectiveness and safety of montelukast in asthma. What are the conclusions from clinical trials and meta-analyses? Drug Des. Devel. Ther. 2014, 26, 839–850. [Google Scholar] [CrossRef]
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention: Updated 2020. Available online: https://www.ginasthma.org (accessed on 30 November 2020).
- Benard, B.; Bastien, V.; Vinet, B.; Yang, R.; Krajinovic, M.; Ducharme, F.M. Neuropsychiatric adverse drug reactions in children initiated on montelukast in real-life practice. Eur. Respir. J. 2017, 50, 1700148. [Google Scholar] [CrossRef] [PubMed]
- Bygdell, M.; Brunlöf, G.; Wallerstedt, S.M.; Kindblom, J.M. Psychiatric adverse drug reactions reported during a 10-year period in the Swedish pediatric population. Pharmacoepidemiol. Drug Saf. 2012, 21, 79–86. [Google Scholar] [CrossRef]
- Wallerstedt, S.M.; Brunlöf, G.; Sundström, A.; Eriksson, A.L. Montelukast and psychiatric disorders in children. Pharmacoepidemiol. Drug Saf. 2009, 18, 858–864. [Google Scholar] [CrossRef]
- Haarman, M.G.; van Hunsel, F.; de Vries, T.W. Adverse drug reactions of montelukast in children and adults. Pharmacol. Res. Perspect. 2017, 5, e00341. [Google Scholar] [CrossRef] [PubMed]
- FDA Drug Safety Communications: FDA Requires Boxed Warning about Serious Mental Health Side Effects for Asthma and Allergy Drug Montelukast (Singulair); Advises Restricting Use for Allergic Rhinitis. 4 March 2020. Available online: www.fda.gov/media/135840/download (accessed on 23 April 2020).
- Glockler-Lauf, S.D.; Finkelstein, Y.; Zhu, J.; Feldman, L.Y.; To, T. Montelukast and Neuropsychiatric Events in Children with Asthma: A Nested Case-Control Study. J. Pediatr. 2019, 209, 176–182.e4. [Google Scholar] [CrossRef]
- Wu, T.Y.; Majeed, A.; Kuo, K.N. An overview of the healthcare system in Taiwan. Lond. J. Prim. Care 2010, 3, 115–119. [Google Scholar] [CrossRef]
- Jager, K.J.; Zoccali, C.; MacLeod, A.; Dekker, F.W. Confounding: What it is and how to deal with it. Kidney Int. 2008, 73, 256–260. [Google Scholar] [CrossRef]
- Cravedi, E.; Deniau, E.; Giannitelli, M.; Xavier, J.; Hartmann, A.; Cohen, D. Tourette syndrome and other neurodevelopmental disorders: A comprehensive review. Child Adolesc. Psychiatry Ment. Health 2017, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Hung, Y.T.; Chuang, Y.L.; Chen, Y.J.; Weng, W.S.; Liu, J.S. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J. Health Manag. 2006, 4, 1–22. [Google Scholar]
- Idova, G.; Cheido, M.; Devoino, L. Modulation of the immune response by changing neuromediator systems activity under stress. Int. J. Immunopharmacol. 1997, 19, 535–540. [Google Scholar] [CrossRef]
- Hoekstra, P.J.; Steenhuis, M.P.; Kallenberg, C.G.; Minderaa, R.B. Association of small life events with self reports of tic severity in pediatric and adult tic disorder patients: A prospective longitudinal study. J. Clin. Psychiatry 2004, 65, 426–431. [Google Scholar] [CrossRef]
- Brodlie, M.; Gupta, A.; Rodriguez-Martinez, C.E.; Castro-Rodriguez, J.A.; Ducharme, F.M.; McKean, M.C. Leukotriene receptor antagonists as maintenance or intermittent treatment in pre-school children with episodic viral wheeze. Paediatr. Respir. Rev. 2016, 17, 57–59. [Google Scholar] [CrossRef]
- Ali, M.M.; O’Brien, C.E.; Cleves, M.A.; Martin, B.C. Exploring the possible association between montelukast and neuropsychiatric events among children with asthma: A matched nested case-control study. Pharmacoepidemiol. Drug Saf. 2015, 24, 435–545. [Google Scholar] [CrossRef]
- Schumock, G.T.; Lee, T.A.; Joo, M.J.; Valuck, R.J.; Stayner, L.T.; Gibbons, R.D. Association between leukotriene-modifying agents and suicide: What is the evidence? Drug Saf. 2011, 34, 533–544. [Google Scholar] [CrossRef]
- Yilmaz Bayer, O.; Turktas, I.; Ertoy Karagol, H.I.; Soysal, S.; Yapar, D. Neuropsychiatric adverse drug reactions induced by montelukast impair the quality of life in children with asthma. J. Asthma 2022, 59, 580–589. [Google Scholar] [CrossRef]
- Stuart, F.A.; Segal, T.Y.; Keady, S. Adverse psychological effects of corticosteroids in children and adolescents. Arch. Dis. Child. 2005, 90, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Okunishi, K.; Peters-Golden, M. Leukotrienes and airway inflammation. Biochim. Biophys. Acta 2011, 1810, 1096–1102. [Google Scholar] [CrossRef]
- Biber, N.; Toklu, H.Z.; Solakoglu, S.; Gultomruk, M.; Hakan, T.; Berkman, Z.; Dulger, F.G. Cysteinyl-leukotriene receptor antagonist montelukast decreases blood-brain barrier permeability but does not prevent oedema formation in traumatic brain injury. Brain Inj. 2009, 23, 577–584. [Google Scholar] [CrossRef]
- Marschallinger, J.; Schäffner, I.; Klein, B.; Gelfert, R.; Rivera, F.J.; Illes, S.; Grassner, L.; Janssen, M.; Rotheneichner, P.; Schmuckermair, C.; et al. Structural and functional rejuvenation of the aged brain by an approved anti-asthmatic drug. Nat. Commun. 2015, 6, 8466. [Google Scholar] [CrossRef] [PubMed]
- Massoumi, R.; Sjölander, A. The role of leukotriene receptor signaling in inflammation and cancer. Sci. World J. 2007, 7, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Barré, J.; Sabatier, J.M.; Annweiler, C. Montelukast Drug May Improve COVID-19 Prognosis: A Review of Evidence. Front. Pharmacol. 2020, 11, 1344. [Google Scholar] [CrossRef] [PubMed]
- Leckman, J.F.; Vaccarino, F.M.; Kalanithi, P.S.; Rothenberger, A. Annotation: Tourette syndrome: A relentless drumbeat-driven by misguided brain oscillations. J. Child Psychol. Psychiatry 2006, 47, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.; Singer, H.S. Tic disorders: Neural circuits, neurochemistry, and neuroimmunology. J. Child Neurol. 2006, 21, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Mell, L.K.; Davis, R.L.; Owens, D. Association between streptococcal infection and obsessive-compulsive disorder, Tourette’s syndrome, and tic disorder. Pediatrics 2005, 116, 56–60. [Google Scholar] [CrossRef]
| Disease | N | % |
|---|---|---|
| Only asthma | 11,285 | 41.8 |
| Only AR | 9490 | 35.2 |
| Only AD | 2304 | 8.5 |
| Asthma and AR | 3503 | 13 |
| Asthma and AD | 66 | 0.3 |
| AR and AD | 167 | 0.6 |
| Asthma and AR and AD | 169 | 0.6 |
| Total | 26,984 | 100 |
| Variables | Study Group 1 | Study Group 2 | Comparison Group | ||
|---|---|---|---|---|---|
| Patients with Asthma or Allergic Rhinitis or Atopic Dermatitis | People without Asthma or Allergic Rhinitis or Atopic Dermatitis and without Receiving LTRA (n = 20,461) | p-Value A | p-Value B | ||
| With Receiving LTRA n = 26,984 | Without Receiving LTRA n = 26,984 | ||||
| Age in years (mean ± SD) | 7.18 ± 3.79 | 7.18 ± 3.79 | 8.11 ± 3.88 | 1.0000 | <0.0001 |
| Gender, male (n (%)) | 15,825 (58.65) | 15,825 (58.65) | 11,093 (54.22) | 1.0000 | <0.0001 |
| ADHD (n (%)) | 911 (3.38) | 501 (1.86) | 396 (1.94) | <0.0001 | 0.5329 |
| Anxiety disorder (n (%)) | 277 (1.03) | 102 (0.38) | 85 (0.42) | <0.0001 | 0.5194 |
| Autism (n (%)) | 171 (0.63) | 99 (0.37) | 64 (0.31) | <0.0001 | 0.3186 |
| Conduct disorder (n (%)) | 17 (0.06) | 6 (0.02) | 4 (0.02) | 0.0218 | 1.0000 |
| Depression (n (%)) | 19 (0.07) | 13 (0.05) | 4 (0.02) | 0.2887 | 0.1027 |
| Epilepsy (n (%)) | 645 (2.39) | 385 (1.43) | 242 (1.18) | <0.0001 | 0.0212 |
| ICS/LABA (n (%)) | 5815 (21.55) | 92 (0.34) | 5 (0.02) | <0.0001 | <0.0001 |
| Intellectual disability (n (%)) | 196 (0.73) | 143 (0.53) | 127 (0.62) | 0.0039 | 0.1931 |
| Learning disorder (n (%)) | 71 (0.26) | 36 (0.13) | 30 (0.15) | 0.0007 | 0.7023 |
| OCD (n (%)) | 35 (0.13) | 18 (0.07) | 13 (0.06) | 0.0195 | 0.8935 |
| Sleep disorder (n (%)) | 270 (1.00) | 114 (0.42) | 55 (0.27) | <0.0001 | 0.0054 |
| Urbanization (n (%)) | Level 1: 16,392 (60.75) Level 2: 8986 (33.30) Level 3: 1606 (5.95) | Level 1: 16,310 (60.44) Level 2: 8761 (32.47) Level 3: 1913 (7.09) | Level 1: 11,111 (54.30) Level 2: 7579 (37.04) Level 3: 1771 (8.66) | <0.0001 | <0.0001 |
| Outcome | Study Group 1 | Study Group 2 | Comparison Group |
|---|---|---|---|
| Patients with Asthma or Allergic Rhinitis or Atopic Dermatitis | People without Asthma or Allergic Rhinitis or Atopic Dermatitis and without Receiving LTRA n = 20,461 | ||
| With Receiving LTRA n = 26,984 | Without Receiving LTRA n = 26,984 | ||
| Tourette syndrome (n(%)) | 868 (3.22) | 606 (2.25) | 125 (0.61) |
| Crude HR (95%CI) | 1.523 (1.373, 1.689) *** | 1 | |
| Adjusted HR (95%CI) | 1.376 (1.232, 1.536) *** | 1 | |
| Crude HR (95%CI) | 3.458 (2.852, 4.192) *** | 1 | |
| Adjusted HR (95%CI) | 2.962 (2.440, 3.597) *** | 1 | |
| Outcome | Study Group 1 | Study Group 2 |
|---|---|---|
| Patients with Asthma or Allergic Rhinitis or Atopic Dermatitis | ||
| With Receiving LTRA n = 26,984 | Without Receiving LTRA n = 26,984 | |
| Asthma only | n = 11,285 | n = 5356 |
| Tourette syndrome (n (%)) | 329 (2.92) | 105 (1.96) |
| Crude HR (95%CI) | 1.557 (1.249, 1.939) *** p < 0.0001 | 1 |
| Adjusted HR (95%CI) | 1.484 (1.184, 1.861) *** p = 0.0006 | 1 |
| Allergic rhinitis only | n = 9490 | n = 16,717 |
| Tourette syndrome (n (%)) | 308 (3.25) | 409 (2.45) |
| Crude HR (95%CI) | 1.459 (1.258, 1.692) *** p < 0.0001 | 1 |
| Adjusted HR (95%CI) | 1.302 (1.104, 1.535) ** p = 0.0017 | 1 |
| Atopic dermatitis only | n = 2304 | n = 3357 |
| Tourette syndrome (n (%)) | 88 (3.76) | 57 (1.70) |
| Crude HR (95%CI) | 2.655 (1.896, 3.718) *** p < 0.0001 | 1 |
| Adjusted HR (95%CI) | 2.350 (1.648, 3.349) *** p < 0.0001 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, M.-L.; Lin, H.-C.; Yen, C.-H.; Ku, J.-T.; Sung, S.-Y.; Chang, H. Increased Risk of Tourette Syndrome with Leukotriene Modifier Use in Children with Allergic Diseases and Asthma: A Nationwide Population-Based Study. Children 2022, 9, 1607. https://doi.org/10.3390/children9111607
Tsai M-L, Lin H-C, Yen C-H, Ku J-T, Sung S-Y, Chang H. Increased Risk of Tourette Syndrome with Leukotriene Modifier Use in Children with Allergic Diseases and Asthma: A Nationwide Population-Based Study. Children. 2022; 9(11):1607. https://doi.org/10.3390/children9111607
Chicago/Turabian StyleTsai, Min-Lan, Hsiu-Chen Lin, Chiung-Hui Yen, Jung-Tzu Ku, Shian-Ying Sung, and Hsi Chang. 2022. "Increased Risk of Tourette Syndrome with Leukotriene Modifier Use in Children with Allergic Diseases and Asthma: A Nationwide Population-Based Study" Children 9, no. 11: 1607. https://doi.org/10.3390/children9111607
APA StyleTsai, M.-L., Lin, H.-C., Yen, C.-H., Ku, J.-T., Sung, S.-Y., & Chang, H. (2022). Increased Risk of Tourette Syndrome with Leukotriene Modifier Use in Children with Allergic Diseases and Asthma: A Nationwide Population-Based Study. Children, 9(11), 1607. https://doi.org/10.3390/children9111607






