Characterization of Rotavirus Infection in Hospitalized Children under 5 with Acute Gastroenteritis 5 Years after Introducing the Rotavirus Vaccines in South Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Research Method
2.2.1. Rotavirus Antigen Detection
2.2.2. Specimen Preservation and Genotype Preanalysis
2.2.3. Rotavirus Genotype Test
2.2.4. VE against RV Hospitalization
2.3. Statistical Analysis
3. Results
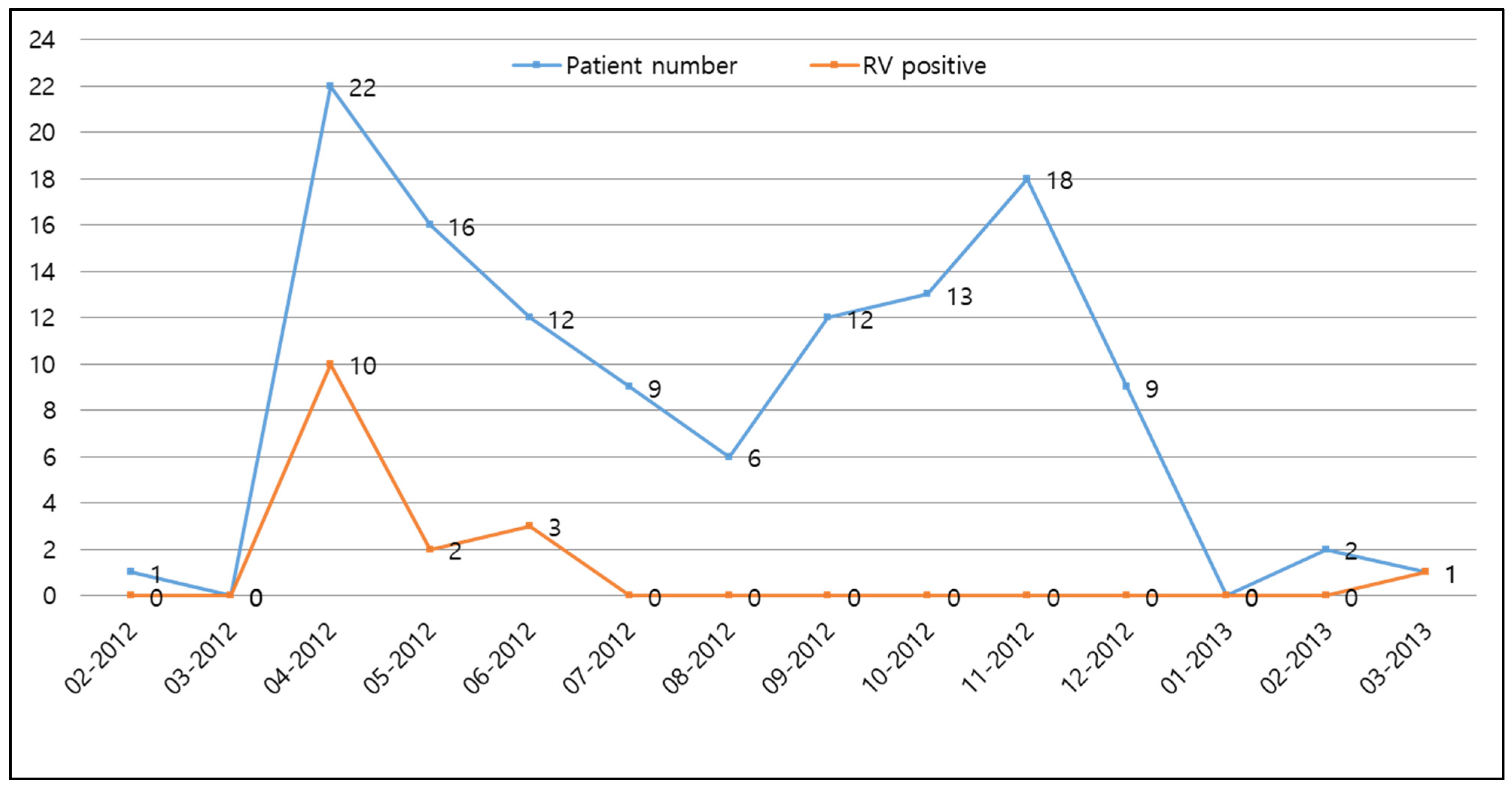
3.1. Demographics and Prevalence of Rotavirus Infection
3.2. Relationship between Rotavirus Infection and Vaccination and VE after Rotavirus Vaccination
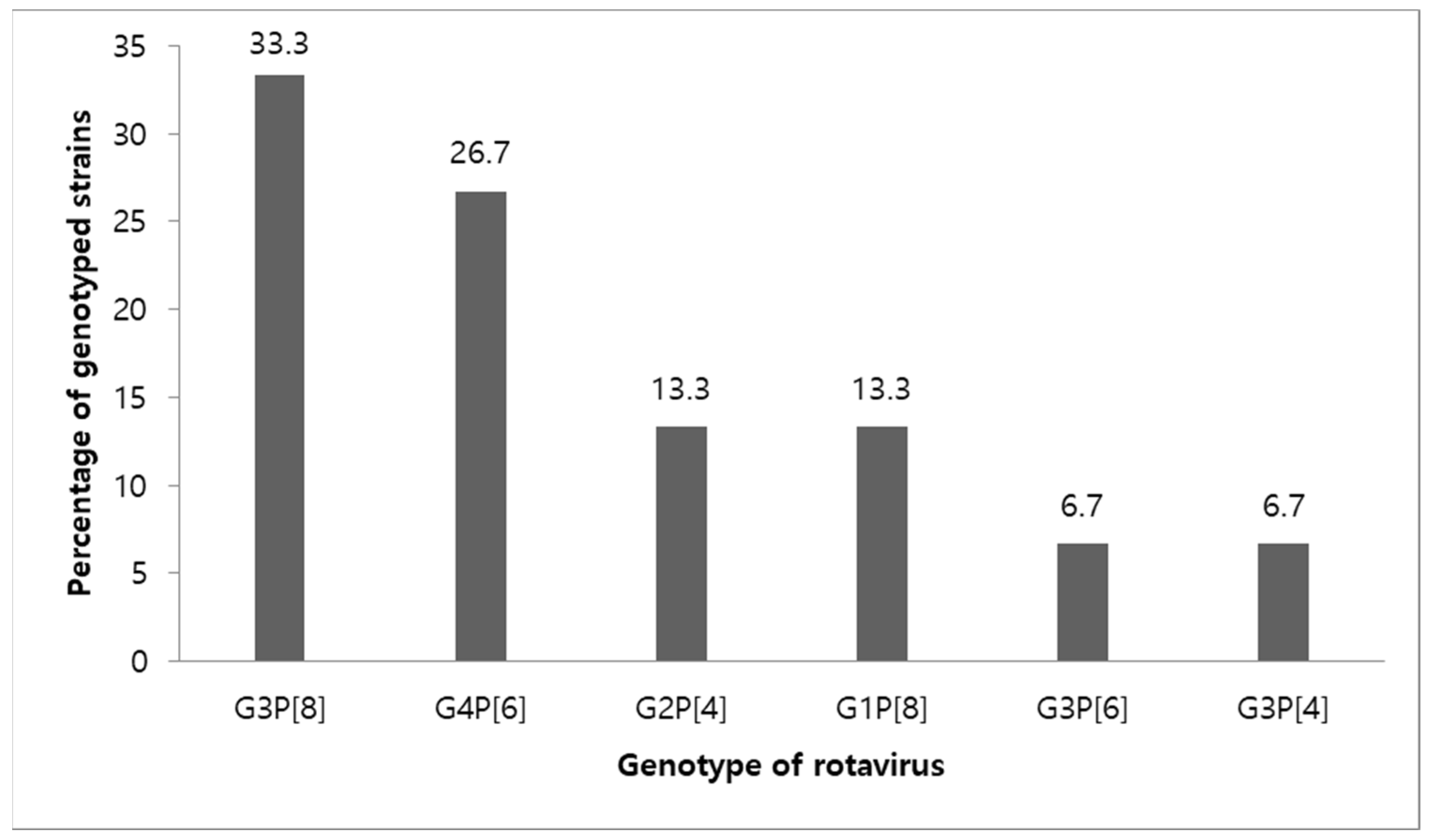
3.3. Genotypes of Rotavirus
3.4. Relationship between Rotavirus Infection and Breastfeeding and between Rotavirus Infection and the Presence of Siblings in Rotavirus Gastroenteritis Children with Hospitalization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Troeger, C.; Blacker, B.F.; Khalil, I.A.; Rao, P.C.; Cao, S.; Zimsen, S.R.; Albertson, S.B.; Stanaway, J.D.; Deshpande, A.; Abebe, Z.; et al. Estimates of the Global, Regional, and National Morbidity, Mortality, and Aetiologies of Diarrhoea in 195 Countries: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.O.; Kilgore, P.; Kim, J.S.; Nyambat, B.; Kim, J.; Suh, H.S.; Yoon, Y.; Jang, S.; Chang, C.; Choi, S.; et al. Molecular Epidemiological Profile of Rotavirus in South Korea, July 2002 through June 2003: Emergence of G4P[6] and G9P[8] Strains. J. Infect. Dis. 2005, 192, S57–S63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadiq, A.; Bostan, N.; Yinda, K.C.; Naseem, S.; Sattar, S. Rotavirus: Genetics, Pathogenesis and Vaccine Advances. Rev. Med. Virol. 2018, 28, e2003. [Google Scholar] [CrossRef] [PubMed]
- Cates, J.E.; Amin, A.B.; Tate, J.E.; Lopman, B.; Parashar, U. Do Rotavirus Strains Affect Vaccine Effectiveness? A Systematic Review and Meta-Analysis. Pediatr. Infect. Dis. J. 2021, 40, 1135–1143. [Google Scholar] [CrossRef]
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T.; Armah, G.; Bines, J.E.; Brewer, T.G.; Colombara, D.V.; et al. Rotavirus Vaccination and the Global Burden of Rotavirus Diarrhea among Children Younger Than 5 Years. JAMA Pediatr. 2018, 172, 958–965. [Google Scholar] [CrossRef] [Green Version]
- Burnett, E.; Parashar, U.D.; Tate, J.E. Real-World Effectiveness of Rotavirus Vaccines, 2006–19: A Literature Review and Meta-Analysis. Lancet Glob. Health 2020, 8, e1195–e1202. [Google Scholar] [CrossRef]
- Leshem, E.; Lopman, B.; Glass, R.; Gentsch, J.; Bányai, K.; Parashar, U.; Patel, M. Distribution of Rotavirus Strains and Strain-Specific Effectiveness of the Rotavirus Vaccine after Its Introduction: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2014, 14, 847–856. [Google Scholar] [CrossRef]
- The Korean Pediatric Society. Rotavirus Vaccine. In Immunization Guideline; Lee, H., Ed.; The Korean Pediatric Society: Seoul, Korea, 2012; pp. 184–194. [Google Scholar]
- Chung, J.Y.; Kim, M.S.; Jung, T.W.; Kim, S.J.; Kang, J.H.; Han, S.B.; Kim, S.Y.; Rhim, J.W.; Kim, H.M.; Park, J.H.; et al. Detection of Rotavirus Genotypes in Korea 5 Years after the Introduction of Rotavirus Vaccines. J. Korean Med. Sci. 2015, 30, 1471–1475. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, H.S.; Kim, H.S.; Kim, J.S.; Song, W.; Lee, K.M.; Lee, S.; Park, K.U.; Lee, W.; Hong, Y.J. Evaluation of an Immunochromatographic Assay for the Rapid and Simultaneous Detection of Rotavirus and Adenovirus in Stool Samples. Ann. Lab. Med. 2014, 34, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.S.; Lim, J.; Sohn, Y.H.; Kim, S.Y. Incidence, Clinical Characteristics, and Genotype Distribution of Rotavirus in a Neonatal Intensive Care Unit 5 Years After Introducing Rotavirus Vaccine. Front. Pediatr. 2022, 10, 850839. [Google Scholar] [CrossRef]
- Gouvea, V.; Glass, R.I.; Woods, P.; Taniguchi, K.; Clark, H.F.; Forrester, B.; Fang, Z.Y. Polymerase Chain Reaction Amplification and Typing of Rotavirus Nucleic Acid from Stool Specimens. J. Clin. Microbiol. 1990, 28, 276–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentsch, J.R.; Glass, R.I.; Woods, P.; Gouvea, V.; Gorziglia, M.; Flores, J.; Das, B.K.; Bhan, M.K. Identification of Group A Rotavirus Gene 4 Types by Polymerase Chain Reaction. J. Clin. Microbiol. 1992, 30, 1365–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esona, M.D.; McDonald, S.; Kamili, S.; Kerin, T.; Gautam, R.; Bowen, M.D. Comparative Evaluation of Commercially Available Manual and Automated Nucleic Acid Extraction Methods for Rotavirus RNA Detection in Stools. J. Virol. Methods 2013, 194, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Ciarlet, M.; McDonald, S.M.; Attoui, H.; Bányai, K.; Brister, J.R.; Buesa, J.; Esona, M.D.; Estes, M.K.; Gentsch, J.R.; et al. Uniformity of Rotavirus Strain Nomenclature Proposed by the Rotavirus Classification Working Group (RCWG). Arch. Virol. 2011, 156, 1397–1413. [Google Scholar] [CrossRef] [Green Version]
- Lewnard, J.A.; Tedijanto, C.; Cowling, B.J.; Lipsitch, M. Measurement of Vaccine Direct Effects Under the Test-Negative Design. Am. J. Epidemiol. 2018, 187, 2686–2697. [Google Scholar] [CrossRef]
- Jackson, M.L.; Nelson, J.C. The Test-Negative Design for Estimating Influenza Vaccine Effectiveness. Vaccine 2013, 31, 2165–2168. [Google Scholar] [CrossRef]
- Burnett, E.; Parashar, U.D.; Tate, J.E. Global Impact of Rotavirus Vaccination on Diarrhea Hospitalizations and Deaths among Children <5 Years Old: 2006–2019. J. Infect. Dis. 2020, 222, 1731–1739. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hong, S.K.; Lee, S.G.; Suh, C.I.; Park, S.W.; Lee, J.H.; Kim, J.H.; Kim, D.S.; Kim, H.M.; Jang, Y.T.; et al. Human Rotavirus Genotypes in Hospitalized Children, South Korea, April 2005 to March 2007. Vaccine 2009, 27, F97–F101. [Google Scholar] [CrossRef]
- Leshem, E.; Moritz, R.E.; Curns, A.T.; Zhou, F.; Tate, J.E.; Lopman, B.A.; Parashar, U.D. Rotavirus Vaccines and Health Care Utilization for Diarrhea in the United States (2007–2011). Pediatrics 2014, 134, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.O.; Chang, J.Y.; Shin, S.; Moon, J.S.; Ko, J.S. Changing Distribution of Age, Clinical Severity, and Genotypes of Rotavirus Gastroenteritis in Hospitalized Children after the Introduction of Vaccination: A Single Center Study in Seoul between 2011 and 2014. BMC Infect. Dis. 2016, 16, 287. [Google Scholar] [CrossRef]
- Ansari, S.A.; Springthorpe, V.S.; Sattar, S.A. Survival and Vehicular Spread of Human Rotaviruses: Possible Relation to Seasonality of Outbreaks. Rev. Infect. Dis. 1991, 13, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-R.; Chae, S.-J.; Jung, S.; Choi, W.; Han, M.-G.; Yoo, C.-K.; Lee, D.-Y. Trends in Acute Viral Gastroenteritis among Children Aged ≤5 Years through the National Surveillance System in South Korea, 2013–2019. J. Med. Virol. 2021, 93, 4875–4882. [Google Scholar] [CrossRef] [PubMed]
- Desselberger, U. Differences of Rotavirus Vaccine Effectiveness by Country: Likely Causes and Contributing Factors. Pathogens 2017, 6, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus Infection. Nat. Rev. Dis. Prim. 2017, 3, 17083. [Google Scholar] [CrossRef] [Green Version]
- Estes, M.K.; Greenberg, H. Rotaviruses. In Fields Virology; Knipe, D., Howley, P., Eds.; Wolters Kluwer/Lippincott, Williams and Wilkins: Philadelphia, PA, USA, 2013; pp. 1347–1401. [Google Scholar]
- Moon, S.-S.; Wang, Y.; Shane, A.L.; Nguyen, T.; Ray, P.; Dennehy, P.; Baek, L.J.; Parashar, U.; Glass, R.I.; Jiang, B. Inhibitory Effect of Breast Milk on Infectivity of Live Oral Rotavirus Vaccines. Pediatr. Infect. Dis. J. 2010, 29, 919–923. [Google Scholar] [CrossRef] [Green Version]
- Trang, N.V.; Braeckman, T.; Lernout, T.; Hau, V.T.B.; Anh, L.T.K.; Luan, L.T.; Van Damme, P.; Anh, D.D. Prevalence of Rotavirus Antibodies in Breast Milk and Inhibitory Effects to Rotavirus Vaccines. Hum. Vaccin. Immunother. 2014, 10, 3681–3687. [Google Scholar] [CrossRef] [Green Version]
- Rongsen-Chandola, T.; Strand, T.A.; Goyal, N.; Flem, E.; Rathore, S.S.; Arya, A.; Winje, B.A.; Lazarus, R.; Shanmugasundaram, E.; Babji, S.; et al. Effect of Withholding Breastfeeding on the Immune Response to a Live Oral Rotavirus Vaccine in North Indian Infants. Vaccine 2014, 32, A134–A139. [Google Scholar] [CrossRef] [Green Version]
- WHO. Rotavirus Vaccines: WHO Position Paper—July 2021. Wkly. Epidemiol. Rec. 2021, 96, 219–301. [Google Scholar]
- Krawczyk, A.; Lewis, M.G.; Venkatesh, B.T.; Nair, S.N. Effect of Exclusive Breastfeeding on Rotavirus Infection among Children. Indian J. Pediatr. 2016, 83, 220–225. [Google Scholar] [CrossRef]
- Weinberg, R.J.; Tipton, G.; Klish, W.J.; Brown, M.R. Effect of Breastfeeding on Morbidity in Rotavirus Gastroenteritis. Pediatrics 1984, 74, 250–253. [Google Scholar] [CrossRef]
- Yolken, R.H.; Peterson, J.A.; Vonderfecht, S.L.; Fouts, E.T.; Midthun, K.; Newburg, D.S. Human Milk Mucin Inhibits Rotavirus Replication and Prevents Experimental Gastroenteritis. J. Clin. Investig. 1992, 90, 1984–1991. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, B.; Zhu, S.; Chen, J. No Direct Correlation between Rotavirus Diarrhea and Breast Feeding: A Meta-Analysis. Pediatr. Neonatol. 2018, 59, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitzer, V.E.; Patel, M.M.; Lopman, B.A.; Viboud, C.; Parashar, U.D.; Grenfell, B.T. Modeling Rotavirus Strain Dynamics in Developed Countries to Understand the Potential Impact of Vaccination on Genotype Distributions. Proc. Natl. Acad. Sci. USA 2011, 108, 19353–19358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, T.L.; Marques da Silva, M.F.; Goméz, M.M.; Resque, H.R.; Ichihara, M.Y.T.; Volotão, E.d.M.; Leite, J.P.G. Evidence of Vaccine-Related Reassortment of Rotavirus, Brazil, 2008–2010. Emerg. Infect. Dis. 2013, 19, 1843–1846. [Google Scholar] [CrossRef]
- Bucardo, F.; Rippinger, C.M.; Svensson, L.; Patton, J.T. Vaccine-Derived NSP2 Segment in Rotaviruses from Vaccinated Children with Gastroenteritis in Nicaragua. Infect. Genet. Evol. 2012, 12, 1282–1294. [Google Scholar] [CrossRef] [Green Version]
- Paul, A.; Gladstone, B.P.; Mukhopadhya, I.; Kang, G. Rotavirus Infections in a Community Based Cohort in Vellore, India. Vaccine 2014, 32, A49–A54. [Google Scholar] [CrossRef]
- Poelaert, D.; Pereira, P.; Gardner, R.; Standaert, B.; Benninghoff, B. A Review of Recommendations for Rotavirus Vaccination in Europe: Arguments for Change. Vaccine 2018, 36, 2243–2253. [Google Scholar] [CrossRef]
- Inns, T.; Trindall, A.; Dunling-Hall, S.; Shankar, A.G. Introduction of a New Rotavirus Vaccine: Initial Results of Uptake and Impact on Laboratory Confirmed Cases in Anglia and Essex, United Kingdom, July 2015. Hum. Vaccin. Immunother. 2016, 12, 1040–1044. [Google Scholar] [CrossRef] [Green Version]
- Paulke-Korinek, M.; Kundi, M.; Rendi-Wagner, P.; de Martin, A.; Eder, G.; Schmidle-Loss, B.; Vecsei, A.; Kollaritsch, H. Herd Immunity after Two Years of the Universal Mass Vaccination Program against Rotavirus Gastroenteritis in Austria. Vaccine 2011, 29, 2791–2796. [Google Scholar] [CrossRef]
- Prelog, M.; Gorth, P.; Zwazl, I.; Kleines, M.; Streng, A.; Zlamy, M.; Heinz-Erian, P.; Wiedermann, U. Universal Mass Vaccination against Rotavirus: Indirect Effects on Rotavirus Infections in Neonates and Unvaccinated Young Infants Not Eligible for Vaccination. J. Infect. Dis. 2016, 214, 546–555. [Google Scholar] [CrossRef] [Green Version]
- Sabbe, M.; Berger, N.; Blommaert, A.; Ogunjimi, B.; Grammens, T.; Callens, M.; Van Herck, K.; Beutels, P.; Van Damme, P.; Bilcke, J. Sustained Low Rotavirus Activity and Hospitalisation Rates in the Post-Vaccination Era in Belgium, 2007 to 2014. Eurosurveillance 2016, 21, 30273. [Google Scholar] [CrossRef] [PubMed]
- Shono, A.; Kondo, M. Factors That Affect Voluntary Vaccination of Children in Japan. Vaccine 2015, 33, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Funk, S.; Kucharski, A.J. Bias Correction Methods for Test-Negative Designs in the Presence of Misclassification. Epidemiol. Infect. 2020, 148, e216. [Google Scholar] [CrossRef] [PubMed]

| Demographics | Number of Patients (%) | p-Value | |
|---|---|---|---|
| RV Ag-Positive | RV Ag-Negative | ||
| age (months) | 0.442 | ||
| 1–11 months | 4 (7.7) | 48 (92.3) | |
| 12–23 months | 5 (14.3) | 30 (85.7) | |
| 24–35 months | 3 (18.7) | 13 (81.3) | |
| 36–47 months | 3 (27.3) | 8 (72.7) | |
| 48–59 months | 1 (14.3) | 6 (85.7) | |
| gender | 0.078 | ||
| male | 12 (18.2) | 54 (81.8) | |
| female | 4 (7.3) | 51 (92.7) | |
| gestational age | 0.224 | ||
| ≥37 weeks | 16 (14.3) | 96 (85.7) | |
| <37 weeks | 0 (0.0) | 9 (100) | |
| birth weight | 0.327 | ||
| >2500 g | 16 (13.9) | 99 (86.1) | |
| ≤2500 g | 0 (0.0) | 6 (100) | |
| delivery | 0.928 | ||
| normal delivery | 11 (13.4) | 71 (86.6) | |
| cesarean section | 5 (12.8) | 34 (87.2) | |
| presence of sibling | <0.001 * | ||
| presence | 14 (25.5) | 41 (74.5) | |
| absence | 2 (3.0) | 64 (96.7) | |
| breastfeeding | 0.774 | ||
| yes | 14 (13.6) | 89 (86.4) | |
| no | 2 (11.1) | 16 (88.9) | |
| total | 16 (13.2) | 105 (86.8) | |
| RV Vaccination Status | p-Value | |||
|---|---|---|---|---|
| Complete V | Incomplete V | Non-V | ||
| Number of patients (%) | 65 (54.6) | 12 (10.1) | 42 (35.3) | |
| RV Ag | 0.002 * | |||
| RV Ag-positive | 2 (3.1) | 2 (16.7) | 11 (26.2) | |
| RV Ag-negative | 63 (96.9) | 10 (83.3) | 31 (73.8) | |
| RV Vaccination Status | p-Value | |||
|---|---|---|---|---|
| Complete V | Incomplete V | Non-V | ||
| Number of Patient (%) | 65 (54.6) | 12 (10.1) | 42 (35.3) | |
| breastfeeding | 0.172 | |||
| yes | 56 (86.2) | 12 (100) | 33 (78.6) | |
| no | 9 (13.8) | 0 (0) | 9 (21.4) | |
| presence of siblings | <0.001 * | |||
| presence | 18 (27.7) | 8 (66.7) | 28 (66.7) | |
| absence | 47 (72.3) | 4 (33.3) | 14 (33.3) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, H.S.; Sohn, Y.-H.; Chae, J.D.; Lim, J.; Kim, S.Y. Characterization of Rotavirus Infection in Hospitalized Children under 5 with Acute Gastroenteritis 5 Years after Introducing the Rotavirus Vaccines in South Korea. Children 2022, 9, 1633. https://doi.org/10.3390/children9111633
Yoon HS, Sohn Y-H, Chae JD, Lim J, Kim SY. Characterization of Rotavirus Infection in Hospitalized Children under 5 with Acute Gastroenteritis 5 Years after Introducing the Rotavirus Vaccines in South Korea. Children. 2022; 9(11):1633. https://doi.org/10.3390/children9111633
Chicago/Turabian StyleYoon, Hye Sun, Yong-Hak Sohn, Jeong Don Chae, Jiseun Lim, and Seung Yeon Kim. 2022. "Characterization of Rotavirus Infection in Hospitalized Children under 5 with Acute Gastroenteritis 5 Years after Introducing the Rotavirus Vaccines in South Korea" Children 9, no. 11: 1633. https://doi.org/10.3390/children9111633






