Recurrent Fever with Oral Lesions in Egyptian Children: A Familial Mediterranean Fever Diagnosis Not to Be Missed
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Laboratory Investigations
2.3. Statistical Analysis
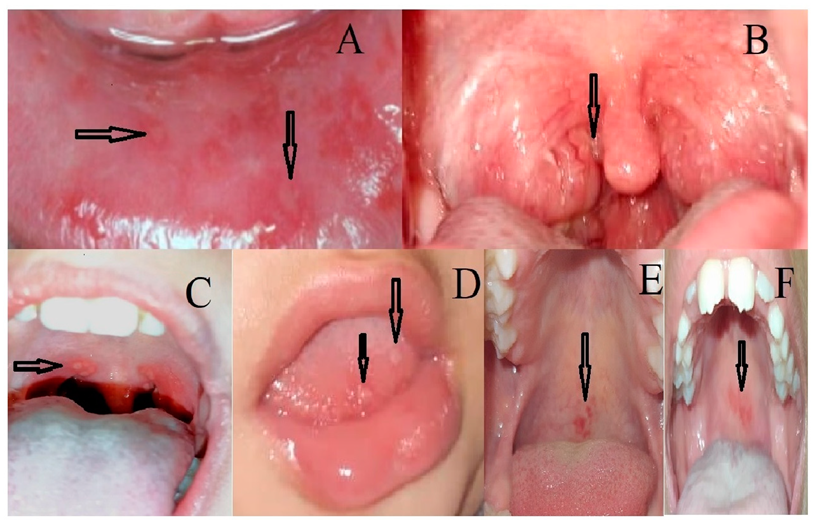
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soon, G.S.; Laxer, R.M. Approach to recurrent fever in childhood. Can. Fam. Physician 2017, 63, 756–762. [Google Scholar] [PubMed]
- Tunca, M.; Akar, S.; Onen, F.; Ozdogan, H.; Kasapcopur, O.; Yalcinkaya, F.; Tutar, E.; Ozen, S.; Topaloglu, R.; Yilmaz, E.; et al. Familial Mediterranean fever (FMF) in Turkey: Results of a nationwide multicenter study. Medicine 2005, 84, 1–11. [Google Scholar]
- Maggio, M.C.; Corsello, G. FMF is not always “fever”: From clinical presentation to “treat to target”. Ital. J. Pediatr. 2020, 46, 7. [Google Scholar] [CrossRef] [PubMed]
- Shinar, Y.; Obici, L.; Aksentijevich, I.; Bennetts, B.; Austrup, F.; Ceccherini, I.; Costa, J.M.; De Leener, A.; Gattorno, M.; Kania, U.; et al. Guidelines for the genetic diagnosis of hereditary recurrent fevers. Ann. Rheum. Dis. 2012, 71, 1599–1605. [Google Scholar] [CrossRef]
- Kishida, D.; Nakamura, A.; Yazaki, M.; Tsuchiya-Suzuki, A.; Matsuda, M.; Ikeda, S.-I. Genotype-phenotype correlation in Japanese patients with familial Mediterranean fever: Differences in genotype and clinical features between Japanese and Mediterranean populations. Arthritis Res. Ther. 2014, 16, 439. [Google Scholar] [CrossRef] [Green Version]
- Ben-Chetrit, E.; Yazici, H. Familial Mediterranean fever: Different faces around the world. Clin. Exp. Rheumatol. 2019, 37, S18–S22. [Google Scholar]
- Ayaz, N.A.; Tanatar, A.; Karadağ, G.; Çakan, M.; Keskindemirci, G.; Sönmez, H.E. Comorbidities and phenotype–genotype correlation in children with familial Mediterranean fever. Rheumatol. Int. 2020, 41, 113–120. [Google Scholar] [CrossRef]
- Balta, B.; Erdogan, M.; Kiraz, A.; Akalın, T.; Baştug, F.; Bayram, A. A comprehensive molecular analysis and genotype–phenotype correlation in patients with familial mediterranean fever. Mol. Biol. Rep. 2020, 47, 1835–1843. [Google Scholar] [CrossRef]
- Duşunsel, R.; Dursun, I.; Gündüz, Z.; Poyrazoğlu, M.H.; Gürgöze, M.K.; Dundar, M. Genotype–phenotype correlation in children with familial Mediterranean fever in a Turkish population. Pediatr. Int. 2008, 50, 208–212. [Google Scholar] [CrossRef]
- Ritis, K.; Giaglis, S.; Spathari, N.; Micheli, A.; Zonios, D.; Tzoanopoulos, D.; Deltas, C.C.; Rafail, S.; Mean, R.; Papadopoulos, V.; et al. Non-isotopic RNase cleavage assay for mutation detection in MEFV, the gene responsible for familial Mediterranean fever, in a cohort of Greek patients. Ann. Rheum. Dis. 2004, 63, 438–443. [Google Scholar] [CrossRef] [Green Version]
- Esmeray, P.; Keçeli, T.I.; Tekçiçek, M.; Batu, E.D.; Arıcı, Z.S.; Ünlü, H.K.; Özen, S.; Bilginer, Y. Oral health status in children with familial Mediterranean fever. Turk. J. Pediatr. 2021, 63, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Rigante, D.; Manna, R. Familial Mediterranean fever: Assessing the overall clinical impact and formulating treatment plans. Mediterr. J. Hematol. Infect. Dis. 2019, 11, e2019027. [Google Scholar] [CrossRef] [PubMed]
- Welzel, T.; Ellinghaus, M.; Wildermuth, A.L.; Deschner, N.; Benseler, S.M.; Kuemmerle-Deschner, J.B. Colchicine Effectiveness and Safety in Periodic Fever, Aphthous Stomatitis, Pharyngitis, and Adenitis. Front. Pediatr. 2021, 9, 759664. [Google Scholar] [CrossRef] [PubMed]
- Veres, T.; Amarilyo, G.; Abu Ahmad, S.; Abu Rumi, M.; Brik, R.; Hezkelo, N.; Ohana, O.; Levinsky, Y.; Chodick, G.; Butbul Aviel, Y. Familial Periodic Fever, Aphthous Stomatitis, Pharyngitis and Adenitis Syndrome; Is It a Separate Disease? Front. Pediatr. 2022, 9, 800656. [Google Scholar] [CrossRef]
- Adrovic, A.; Sahin, S.; Barut, K.; Kasapcopur, O. Familial Mediterranean fever and periodic fever, aphthous stomatitis, pharyngitis, and adenitis (PFAPA) syndrome: Shared features and main differences. Rheumatol. Int. 2019, 39, 29–36. [Google Scholar] [CrossRef]
- Manthiram, K.; Lapidus, S.; Edwards, K. Unraveling the pathogenesis of periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis through genetic, immunologic, and microbiologic discoveries: An update. Curr. Opin. Rheumatol. 2017, 29, 493–499. [Google Scholar] [CrossRef]
- Taniuchi, S.; Nishikomori, R.; Iharada, A.; Tuji, S.; Heike, T.; Kaneko, K. MEFV variants in patients with PFAPA syndrome in Japan. Open Rheumatol. J. 2013, 7, 22–25. [Google Scholar] [CrossRef] [Green Version]
- Butbul Aviel, Y.; Harel, L.; Abu Rumi, M. Familial Mediterranean Fever Is Commonly Diagnosed in Children in Israel with Periodic Fever Aphthous Stomatitis, Pharyngitis, and Adenitis Syndrome. J. Pediatr. 2019, 204, 270–274. [Google Scholar] [CrossRef]
- Ben-Chetrit, E.; Touitou, I. Familial Mediterranean fever in the world. Arthritis Care Res. 2009, 61, 1447–1453. [Google Scholar] [CrossRef]
- Çakan, M.; Aktay Ayaz, N.; Keskindemirci, G.; Karadağ, Ş.G.; Tanatar, A.; Sönmez, H.E. Serum amyloid A as a biomarker in differentiating attacks of familial Mediterranean fever from acute febrile infections. Clin. Rheumatol. 2020, 39, 249–253. [Google Scholar] [CrossRef]
- Gattorno, M.; Hofer, M.; Federici, S.; Vanoni, F.; Bovis, F.; Aksentijevich, I.; Anton, J.; Arostegui, J.I.; Barron, K.; Ben-Cherit, E.; et al. Classification criteria for autoinflammatory recurrent fevers. Ann. Rheum. Dis. 2019, 78, 1025–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirkaya, E.; Saglam, C.; Turker, T.; Koné-Paut, I.; Woo, P.; Doglio, M.; Amaryan, G.; Frenkel, J.; Uziel, Y.; Insalaco, A.; et al. Performance of different diagnostic criteria for familial Mediterranean fever in children with periodic fevers: Results from a multicenter international registry. J. Rheumatol. 2015, 43, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Özdel, S.; Özçakar, Z.B.; Kunt, S.Ş.; Elhan, A.H.; Yalçınkaya, F. Late-onset disease is associated with a mild phenotype in children with familial Mediterranean fever. Clin. Rheumatol. 2016, 35, 1837–1840. [Google Scholar] [CrossRef] [PubMed]
- Talaat, H.S.E.-D.; Mohamed, M.F.; El Rifai, N.M.M.; Gomaa, M.A. The expanded clinical profile and the efficacy of colchicine therapy in Egyptian children suffering from familial Mediterranean fever: A descriptive study. Ital. J. Pediatr. 2012, 38, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öztürk, K.; Coşkuner, T.; Baglan, E.; Sönmez, H.E.; Yener, G.O.; Çakmak, F.; Demirkan, F.G.; Tanatar, A.; Karadag, S.G.; Ozdel, S.; et al. Real-Life Data From the Largest Pediatric Familial Mediterranean Fever Cohort. Front. Pediatr. 2022, 9, 1676. [Google Scholar] [CrossRef]
- Yepiskoposyan, L.; Harutyunyan, A. Population genetics of familial Mediterranean fever: A review. Eur. J. Hum. Genet. 2007, 15, 911–916. [Google Scholar] [CrossRef] [Green Version]
- Mansour, A.R.; El-Shayeb, A.; El Habachi, N.; Khodair, M.A.; Elwazzan, D.; Abdeen, N.; Said, M.; Ebaid, R.; ElShahawy, N.; Seif, A.; et al. Molecular patterns of MEFV gene mutations in Egyptian patients with familial Mediterranean fever: A retrospective cohort study. Int. J. Inflamm. 2019, 2019, 2578760. [Google Scholar] [CrossRef] [Green Version]
- Zarouk, W.A.; El-Bassyouni, H.T.; Ramadan, A.; Fayez, A.; Esmaiel, N.N.; Foda, B.M.; Kobiesy, M.M.; Zekry, M.E.; Lotfy, R.S.; Shehata, G.M. Screening of the most common MEFV mutations in a large cohort of Egyptian patients with Familial Mediterranean fever. Gene Rep. 2018, 11, 23–28. [Google Scholar] [CrossRef]
- Talaat, H.S.; Sheba, M.F.; Mohammed, R.H.; Gomaa, M.A.; El Rifaei, N.; Ibrahim, M.F.M. Genotype mutations in Egyptian children with familial mediterranean fever: Clinical profile, and response to Colchicine. Mediterr. J. Rheumatol. 2020, 31, 206. [Google Scholar] [CrossRef]
- Arpacı, A.; Doğan, S.; Erdoğan, H.F.; El, Ç.; Cura, S.E. Presentation of a new mutation in FMF and evaluating the frequency of distribution of the MEFV gene mutation in our region with clinical findings. Mol. Biol. Rep. 2021, 48, 2025–2033. [Google Scholar] [CrossRef]
- Batu, E.D.; Eroğlu, F.K.; Tsoukas, P.; Hausmann, J.S.; Bilginer, Y.; Kenna, M.A.; Licameli, G.R.; Fuhlbrigge, R.C.; Özen, S.; Dedeoğlu, F. Periodic fever, aphthosis, pharyngitis, and adenitis syndrome: Analysis of patients from two geographic areas. Arthritis Care Res. 2016, 68, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Natural history of mevalonate kinase deficiency: A literature review. Pediatr. Rheumatol. 2016, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Kone Paut, I.; Dubuc, M.; Sportouch, J.; Minodier, P.; Garnier, J.M.; Touitou, I. Phenotype–genotype correlation in 91 patients with familial Mediterranean fever reveals a high frequency of cutaneomucous features. Rheumatology 2000, 39, 1275–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wang, W.; Zhong, L.; Pan, J.; Yu, Z.; Jian, S.; Wang, C.; Ma, M.; Tang, X.; Wang, L.; et al. Familial Mediterranean Fever in Chinese Children: A Case Series. Front. Pediatr. 2019, 7, 483. [Google Scholar] [CrossRef] [PubMed]
- Mkrtchyan, N.; Amaryan, G.; Sarkisian, T. Coexistence of PFAPA syndrome and FMF in Armenian children. Pediatr. Rheumatol. 2015, 13, P102. [Google Scholar] [CrossRef] [Green Version]
- Celiksoy, M.H.; Ogur, G.; Yaman, E.; Abur, U.; Fazla, S.; Sancak, R.; Yildiran, A. Could familial Mediterranean fever gene mutations be related to PFAPA syndrome? Pediatr. Allergy Immunol. 2016, 27, 78–82. [Google Scholar] [CrossRef]
- Ozen, S.; Besbas, N.; Bakkaloglu, A.; Yilmaz, E. Pyrin Q148 mutation and familial Mediterranean fever. QJM Int. J. Med. 2002, 95, 332–333. [Google Scholar] [CrossRef] [Green Version]
- Topaloglu, R.; Ozaltin, F.; Yilmaz, E.; Ozen, S.; Balci, B.; Besbas, N.; Bakkaloglu, A. E148Q is a disease-causing MEFV mutation: A phenotypic evaluation in patients with familial Mediterranean fever. Ann. Rheum. Dis. 2005, 64, 750–752. [Google Scholar] [CrossRef] [Green Version]
- Beshlawy, A.E.; Zekri, A.E.R.; Ramadan, M.S.; Selim, Y.M.; Abdel-Salam, A.; Hegazy, M.T.; Ragab, L.; Gaggiano, C.; Cantarini, L.; Ragab, G. Genotype–phenotype associations in familial Mediterranean fever: A study of 500 Egyptian pediatric patients. Clin. Rheumatol. 2022, 41, 1511–1521. [Google Scholar] [CrossRef]
- Cekin, N.; Akyurek, M.E.; Pinarbasi, E.; Ozen, F. MEFV mutations and their relation to major clinical symptoms of Familial Mediterranean Fever. Gene 2017, 626, 9–13. [Google Scholar] [CrossRef]
- Coskun, B.; Kiraz, A.; Sevinc, E.; Baspinar, O.; Cakmak, E. Increased frequency of MEFV genes in patients with epigastric pain syndrome. Balk. J. Med. Genet. 2017, 20, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padeh, S.; Shinar, Y.; Pras, E.; Zemer, D.; Langevitz, P.; Pras, M.; Livneh, A. Clinical and diagnostic value of genetic testing in 216 Israeli children with Familial Mediterranean fever. J. Rheumatol. 2003, 30, 185–190. [Google Scholar] [PubMed]
- Touitou, I. Inheritance of autoinflammatory diseases: Shifting paradigms and nomenclature. J. Med. Genet. 2013, 50, 349–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etem, E.O.; Deveci, S.D.; Erol, D.; Yuce, H.; Elyas, H. Familial Mediterranean fever: A retrospective clinical and molecular study in the East of Anatolia region of Turkey. Open Rheumatol. J. 2010, 4, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kırnaz, B.; Gezgin, Y.; Berdeli, A. MEFV gene allele frequency and genotype distribution in 3230 patients’ analyses by next generation sequencing methods. Gene 2022, 827, 146447. [Google Scholar] [CrossRef]
- Gumus, E. The frequency of MEFV gene mutations and genotypes in Sanliurfa province, South-Eastern region of Turkey, after the Syrian Civil War by using next generation sequencing and report of a Novel Exon 4 Mutation (I423T). J. Clin. Med. 2018, 7, 105. [Google Scholar] [CrossRef] [Green Version]
- Sgouropoulou, V.; Farmaki, E.; Papadopoulos, T.; Tzimouli, V.; Pratsidou-Gertsi, J.; Trachana, M. Sequence analysis in Familial Mediterranean Fever patients with no confirmatory genotype. Rheumatol. Int. 2022, 42, 15–22. [Google Scholar] [CrossRef]
| Eurofever/PRINTO Classification Criteria |
|---|
| Presence of confirmatory MEFV genotype * and at least one among the following: ● Duration of episodes 1–3 days. ● Arthritis ● Chest pain ● Abdominal pain |
| Or |
| Presence of not confirmatory MEFV genotype † and at least two among the following: ● Duration of episodes 1–3 days. ● Arthritis ● Chest pain. ● Abdominal pain |
| Demographic Data n (%) | |
|---|---|
| Age range of diagnosis Mean ± SD (Years) | (10 months–10 years) 3.5 ± 1.55805 |
| Male | 38 (58.5%) |
| Female | 27 (42.5%) |
| Positive family history | 21 (32.3%) |
| Main clinical presentations n (%) | |
| Recurrent Fever | 65 (100%) |
| Recurrent oral lesions | 41 (63%) |
| Recurrent abdominal pain | 20 (31%) |
| Recurrent musculoskeletal pain | 4 (6%) |
| Laboratory data | |
| SAA level Mean ± SD (mg/L) | 162.5 ± 85.78 |
| CRP (mg/dL) | 33 ± 15 |
| Genetic data (Type of mutations) n (%) | |
| Homozygous | 6 (10.7%) |
| Heterozygous | 44 (78.6%) |
| Compound Heterozygous | 6 (10.7%) |
| Genetic data (Common mutations) n (%) 64 mutations in 56 patients | |
| E148Q | 24 (37.5%) |
| M694I | 18 (32.1%) |
| V726A | 13 (20.3%) |
| P369S | 5 (7.8%) |
| M680I(G/A) | 3 (4.7%) |
| R761H | 1 (1.6%) |
| Mutation Type | SAA Mean ± SD | MEFVMutation | SAA Mean ± SD |
|---|---|---|---|
| Homozygous | 193.1 ± 20.61899 | E148Q | 170.9 ± 79.9 |
| Heterozygous | 181.8 ± 74.1403 | M680I | 205.0 ± 8.2 |
| Combined Heterozygous | 194.7 ± 16.01879 | M694I | 211.8 ± 46.1 |
| p-value | 0.849 | V726A | 178.6 ± 20.1 |
| p-value | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omran, A.; Abdelrahman, A.; Mohamed, Y.G.; Abdalla, M.O.; Abdel-Hamid, E.R.; Elfiky, S. Recurrent Fever with Oral Lesions in Egyptian Children: A Familial Mediterranean Fever Diagnosis Not to Be Missed. Children 2022, 9, 1654. https://doi.org/10.3390/children9111654
Omran A, Abdelrahman A, Mohamed YG, Abdalla MO, Abdel-Hamid ER, Elfiky S. Recurrent Fever with Oral Lesions in Egyptian Children: A Familial Mediterranean Fever Diagnosis Not to Be Missed. Children. 2022; 9(11):1654. https://doi.org/10.3390/children9111654
Chicago/Turabian StyleOmran, Ahmed, Ahmed Abdelrahman, Yasmine Gabr Mohamed, Mohamed Osama Abdalla, Eman R. Abdel-Hamid, and Samar Elfiky. 2022. "Recurrent Fever with Oral Lesions in Egyptian Children: A Familial Mediterranean Fever Diagnosis Not to Be Missed" Children 9, no. 11: 1654. https://doi.org/10.3390/children9111654





