Interstitial Lung Disease in Children: “Specific Conditions of Undefined Etiology” Becoming Clearer
Abstract
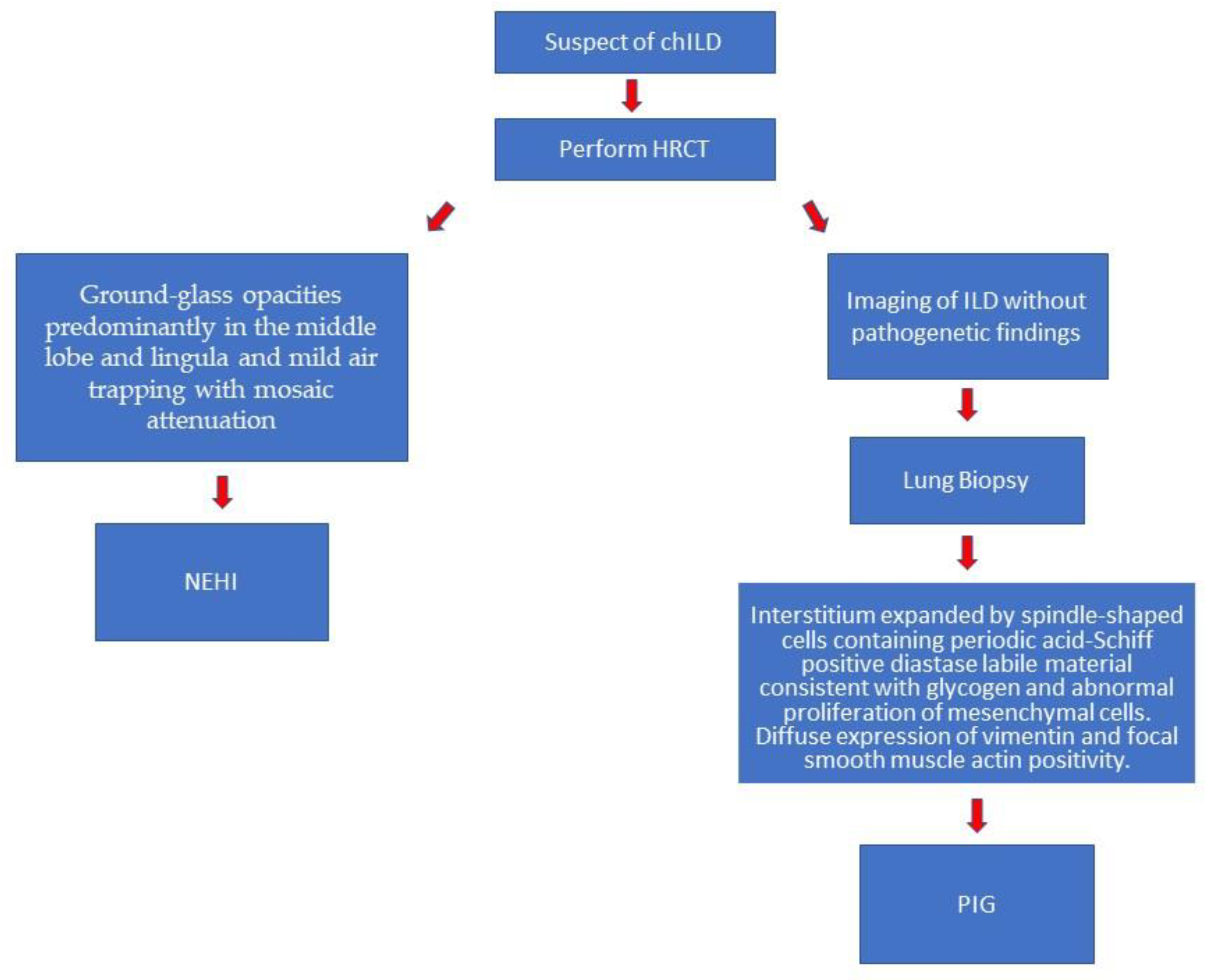
1. Introduction
2. Materials and Methods
3. Results
3.1. Neuroendocrine Cell Hyperplasia of Infancy (NEHI)
| Age at onset | Within the first year | [9,21] | HRCT findings | Specific: ground-glass opacities predominantly in the middle lobe and lingula and mild air-trapping with mosaic attenuation | [24,25,26,27] |
| Etiology | Unknown | [11] | Diagnosis | Clinical and radiological | [2,22,23] |
| Anomalies | Neuroendocrine cells in respiratory bronchioles | [12] | Biopsy | Mild increase of alveolar macrophages and smooth muscle hyperplasia of bronchioles and the presence of NECs within distal airways, marked by immunostains against bombesin and serotonin | [9,28] |
| Lung dynamic alterations | Low tidal volumes, high minute ventilation, low forced vital capacity (FVC). Functional residual capacity, residual volume, and residual volume/total lung capacity are above the norm. Generally, post-bronchodilator measurements do not show improvements | [10,15] | Therapy | Supportive oxygen treatment | [15,32] |
| Signs and symptoms | Chronic tachypnea, hypoxemia (>90%), retractions and crackles (>80%), failure to thrive and developmental delays | [16,17,18,19,20] | Prognosis | Very good, often with a complete recover | [15,33,35] |
3.2. Pulmonary Interstitial Glycogenosis (PIG)
| Age at onset | Neonatal or first months | [7,8,36] | HRCT findings | Not specific: ground-glass opacities, cystic lucencies, both predominantly in posterior lung fields, consolidations, interlobular septal thickening, linear opacities, mosaic attenuation and architectural distortion | [37] |
| Etiology | Unknown | [7,36,37,38] | Diagnosis | Biopsy needed | [37] |
| Anomalies | Spindle-shaped cells containing periodic acid–Schiff positive–diastase labile material consistent with glycogen and abnormal proliferation of mesenchymal cells that expand the interstitium | [36,38,46] | Biopsy | Interstitium expanded by spindle-shaped cells containing periodic acid–Schiff positive–diastase labile material consistent with glycogen and abnormal proliferation of mesenchymal cells. Histopathological pattern represented by patchy or diffuse distribution. Diffuse expression of vimentin and focal smooth muscle actin positivity. In addition to glycogen, droplets of neutral lipid are present. | [8,44,46] |
| Lung dynamic alterations | Mostly obstructive pattern while restrictive pattern is possible but less frequent | Therapy | Oxygen supplementation and systemic corticosteroids | [42] | |
| Signs and symptoms | Tachypnea and hypoxemia. Two thirds of patients require neonatal resuscitation, including non-invasive ventilation and/or invasive mechanical ventilation | [37,40,42] | Prognosis | Variable, half of patients become asymptomatic after 2 or 3 years from the diagnosis | [47,48] |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurland, G.; Deterding, R.R.; Hagood, J.S.; Young, L.R.; Brody, A.S.; Castile, R.G.; Dell, S.; Fan, L.L.; Hamvas, A.; Hilman, B.C.; et al. An Official American Thoracic Society Clinical Practice Guideline: Classification, Evaluation, and Management of Childhood Interstitial Lung Disease in Infancy. Am. J. Respir. Crit. Care Med. 2013, 188, 376–394. [Google Scholar] [CrossRef] [PubMed]
- Nathan, N.; Berdah, L.; Delestrain, C.; Sileo, C.; Clement, A. Interstitial lung diseases in children. Presse Med. 2020, 49, 103909. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.; Jaffe, A.; Young, L.R. Children’s interstitial and diffuse lung disease. Lancet Child Adolesc. Health 2019, 3, 568–577. [Google Scholar] [CrossRef]
- Nathan, N.; Corvol, H.; Amselem, S.; Clement, A. Biomarkers in Interstitial lung diseases. Paediatr. Respir. Rev. 2015, 16, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Guillot, L.; Nathan, N.; Tabary, O.; Thouvenin, G.; Le Rouzic, P.; Corvol, H.; Amselem, S.; Clement, A. Alveolar epithelial cells: Master regulators of lung homeostasis. Int. J. Biochem. Cell Biol. 2013, 45, 2568–2573. [Google Scholar] [CrossRef] [PubMed]
- Nogee, L.M. Interstitial lung disease in newborns. Semin. Fetal Neonatal Med. 2017, 22, 227–233. [Google Scholar] [CrossRef]
- Bush, A.; Griese, M.; Seidl, E.; Kerem, E.; Reu, S.; Nicholson, A.G. Early onset children’s interstitial lung diseases: Discrete entities or manifestations of pulmonary dysmaturity? Paediatr. Respir. Rev. 2019, 30, 65–71. [Google Scholar] [CrossRef]
- Bush, A.; Gilbert, C.; Gregory, J.; Nicholson, A.G.; Semple, T.; Pabary, R. Interstitial lung disease in infancy. Early Hum. Dev. 2020, 150, 105186. [Google Scholar] [CrossRef]
- Deterding, R.R.; Pye, C.; Fan, L.L.; Langston, C. Persistent tachypnea of infancy is associated with neuroendocrine cell hyperplasia. Pediatr. Pulmonol. 2005, 40, 157–165. [Google Scholar] [CrossRef]
- Young, L.R.; Brody, A.S.; Inge, T.H.; Acton, J.D.; Bokulic, R.E.; Langston, C.; Deutsch, G.H. Neuroendocrine cell distribution and frequency distinguish neuroendocrine cell hyperplasia of infancy from other pulmonary disorders. Chest 2011, 139, 1060–1071. [Google Scholar] [CrossRef]
- Young, L.R.; Deutsch, G.H.; Bokulic, R.E.; Brody, A.S.; Nogee, L.M. A Mutation in TTF1/NKX2.1 Is Associated with Familial Neuroendocrine Cell Hyperplasia of Infancy. Chest 2013, 144, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, R.L.; Torday, J.S.; Mu, Q.; Asokananthan, N.; Sikorski, K.A.; Sunday, M.E. Bombesin-like peptides and receptors in normal fetal baboon lung: Roles in lung growth and maturation. Am. J. Physiol. 1999, 277, L1003–L1017. [Google Scholar] [CrossRef] [PubMed]
- Popler, J.; Gower, W.A.; Mogayzel, P.J.; Nogee, L.M.; Langston, C.; Wilson, A.C.; Hay, T.C.; Deterding, R.R. Familial neuroendocrine cell hyperplasia of infancy. Pediatr. Pulmonol. 2010, 45, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Yancheva, S.G.; Velani, A.; Rice, A.; Montero, A.; Hansell, D.M.; Koo, S.; Thia, L.; Bush, A.; Nicholson, A.G. Bombesin staining in neuroendocrine cell hyperplasia of infancy (NEHI) and other childhood interstitial lung diseases (chILD). Histopathology 2015, 67, 501–508. [Google Scholar] [CrossRef]
- Lukkarinen, H.; Pelkonen, A.; Lohi, J.; Malmström, K.; Malmberg, L.P.; Kajosaari, M.; Lindahl, H.; Föhr, A.; Ruuskanen, O.; Mäkelä, M. Neuroendocrine cell hyperplasia of infancy: A prospective follow-up of nine children. Arch. Dis. Child. 2013, 98, 141–144. [Google Scholar] [CrossRef]
- Carr, L.L.; Kern, J.A.; Deutsch, G.H. Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia and Neuroendocrine Hyperplasia of Infancy. Clin. Chest Med. 2016, 37, 579–587. [Google Scholar] [CrossRef]
- Kerby, G.S.; Wagner, B.D.; Popler, J.; Hay, T.C.; Kopecky, C.; Wilcox, S.L.; Quinones, R.R.; Giller, R.H.; Accurso, F.J.; Deterding, R.R. Abnormal infant pulmonary function in young children with neuroendocrine cell hyperplasia of infancy. Pediatr. Pulmonol. 2013, 48, 1008–1015. [Google Scholar] [CrossRef]
- Breuer, O.; Cohen-Cymberknoh, M.; Picard, E.; Bentur, L.; Bar-Yoseph, R.; Shoseyov, D.; Tsabari, R.; Kerem, E.; Hevroni, A. The Use of Infant Pulmonary Function Tests in the Diagnosis of Neuroendocrine Cell Hyperplasia of Infancy. Chest 2021, 160, 1397–1405. [Google Scholar] [CrossRef]
- Liptzin, D.R.; Pickett, K.; Brinton, J.T.; Agarwal, A.; Fishman, M.P.; Casey, A.; Towe, C.T.; Taylor, J.B.; Kurland, G.; Hagood, J.S.; et al. Neuroendocrine Cell Hyperplasia of Infancy. Clinical Score and Comorbidities. Ann. Am. Thorac. Soc. 2020, 17, 724–728. [Google Scholar] [CrossRef]
- Nevel, R.J.; Garnett, E.T.; Schaudies, D.A.; Young, L.R. Growth trajectories and oxygen use in neuroendocrine cell hyperplasia of infancy. Pediatr. Pulmonol. 2018, 53, 656–663. [Google Scholar] [CrossRef]
- Balinotti, J.E.; Maffey, A.; Colom, A.; Roldán, O.; Díaz, W.; Medín, M.; Racimo, M.; Teper, A. Clinical, functional, and computed tomography findings in a cohort of patients with neuroendocrine cell hyperplasia of infancy. Pediatr. Pulmonol. 2021, 56, 1681–1686. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.; Cunningham, S.; de Blic, J.; Barbato, A.; Clement, A.; Epaud, R.; Hengst, M.; Kiper, N.; Nicholson, A.G.; Wetzke, M.; et al. European protocols for the diagnosis and initial treatment of interstitial lung disease in children. Thorax 2015, 70, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.; Gilbert, C.; Gregory, J.; Nicholson, A.G.; Semple, T.; Zampoli, M.; Pabary, R. Pediatric interstitial lung disease. J. Pan Afr. Thorac. Soc. 2021, 2, 18–32. [Google Scholar] [CrossRef]
- Gomes, V.C.C.; Silva, M.C.C.; Maia Filho, J.H.; Daltro, P.; Ramos, S.G.; Brody, A.S.; Marchiori, E. Diagnostic criteria and follow-up in neuroendocrine cell hyperplasia of infancy: A case series. J. Bras. Pneumol. Publicacao Soc. Bras. Pneumol. E Tisilogia 2013, 39, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Brody, A.S.; Guillerman, R.P.; Hay, T.C.; Wagner, B.D.; Young, L.R.; Deutsch, G.H.; Fan, L.L.; Deterding, R.R. Neuroendocrine Cell Hyperplasia of Infancy: Diagnosis with High-Resolution CT. AJR Am. J. Roentgenol 2010, 194, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Semple, T.R.; Ashworth, M.T.; Owens, C.M. Interstitial Lung Disease in Children Made Easier…Well, almost. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2017, 37, 1679–1703. [Google Scholar] [CrossRef]
- Spielberg, D.R.; Brody, A.S.; Baker, M.L.; Woods, J.C.; Towe, C.T. Ground-glass burden as a biomarker in neuroendocrine cell hyperplasia of infancy. Pediatr. Pulmonol. 2019, 54, 822–827. [Google Scholar] [CrossRef]
- Cutz, E. Hyperplasia of pulmonary neuroendocrine cells in infancy and childhood. Semin. Diagn Pathol. 2015, 32, 420–437. [Google Scholar] [CrossRef]
- Wang, B.; Cardenas, M.; Bedoya, M.; Colin, A.A.; Rossi, G.A. Upregulation of neuropeptides and obstructive airway disorder in infancy: A review with focus on post-RSV wheezing and NEHI. Pediatr. Pulmonol. 2021, 56, 1297–1306. [Google Scholar] [CrossRef]
- Mastej, E.J.; DeBoer, E.M.; Humphries, S.M.; Cook, M.C.; Hunter, K.S.; Liptzin, D.R.; Weinman, J.P.; Deterding, R.R. Lung and airway shape in neuroendocrine cell hyperplasia of infancy. Pediatr. Radiol. 2018, 48, 1745–1754. [Google Scholar] [CrossRef]
- Doan, M.L.; Elidemir, O.; Dishop, M.K.; Zhang, H.; Smith, E.O.; Black, P.G.; Deterding, R.R.; Roberts, D.M.; A Al-Salmi, Q.; Fan, L.L. Serum KL-6 differentiates neuroendocrine cell hyperplasia of infancy from the inborn errors of surfactant metabolism. Thorax 2009, 64, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, V.A.; Zanconato, S.; Zamunaro, A.; Carraro, S. Children’s Interstitial and Diffuse Lung Diseases (ChILD) in 2020. Children 2020, 7, 280. [Google Scholar] [CrossRef] [PubMed]
- Houin, P.R.; Deterding, R.R.; Young, L.R. Exacerbations in neuroendocrine cell hyperplasia of infancy are characterized by increased air trapping. Pediatr. Pulmonol. 2016, 51, E9–E12. [Google Scholar] [CrossRef]
- Nevel, R.J.; Garnett, E.T.; Worrell, J.A.; Morton, R.L.; Nogee, L.M.; Blackwell, T.S.; Young, L.R. Persistent Lung Disease in Adults with NKX2.1 Mutation and Familial Neuroendocrine Cell Hyperplasia of Infancy. Ann. Am. Thorac. Soc. 2016, 13, 1299–1304. [Google Scholar] [CrossRef]
- Liptzin, D.R.; Hawkins, S.M.M.; Wagner, B.D.; Deterding, R.R. Sleeping chILD: Neuroendocrine cell hyperplasia of infancy and polysomnography. Pediatr. Pulmonol. 2018, 53, 917–920. [Google Scholar] [CrossRef]
- Canakis, A.M.; Cutz, E.; Manson, D.; O’Brodovich, H. Pulmonary interstitial glycogenosis: A new variant of neonatal interstitial lung disease. Am. J. Respir. Crit. Care Med. 2002, 165, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Seidl, E.; Carlens, J.; Reu, S.; Wetzke, M.; Ley-Zaporozhan, J.; Brasch, F.; Wesselak, T.; Schams, A.; Rauch, D.; Schuch, L.; et al. Pulmonary interstitial glycogenosis-A systematic analysis of new cases. Respir. Med. 2018, 140, 11–20. [Google Scholar] [CrossRef]
- Jeffery, P.K. The Development of Large and Small Airways. Am. J. Respir. Crit. Care Med. 1998, 157, S174–S180. [Google Scholar] [CrossRef]
- Still, G.G.; Li, S.; Wilson, M.; Sammut, P. Persistent Pulmonary Hypertension Without Underlying Cardiac Disease as a Presentation of Pulmonary Interstitial Glycogenosis. Fetal Pediatr. Pathol. 2018, 37, 22–26. [Google Scholar] [CrossRef]
- Cutz, E.; Chami, R.; Dell, S.; Langer, J.; Manson, D. Pulmonary interstitial glycogenosis associated with a spectrum of neonatal pulmonary disorders. Hum. Pathol. 2017, 68, 154–165. [Google Scholar] [CrossRef]
- Weinman, J.P.; White, C.J.; Liptzin, D.R.; Deterding, R.R.; Galambos, C.; Browne, L.P. High-resolution CT findings of pulmonary interstitial glycogenosis. Pediatr. Radiol. 2018, 48, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Liptzin, D.R.; Baker, C.D.; Darst, J.R.; Weinman, J.P.; Dishop, M.K.; Galambos, C.; Brinton, J.T.; Deterding, R.R. Pulmonary interstitial glycogenosis: Diagnostic evaluation and clinical course. Pediatr. Pulmonol. 2018, 53, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.; Vade, A.; Lim-Dunham, J.E.; Masuda, E.; Massarani-Wafai, R. Pulmonary interstitial glycogenosis in the setting of lung growth abnormality: Radiographic and pathologic correlation. Pediatr. Radiol. 2010, 40, 1562–1565. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, G.H.; Young, L.R. Lipofibroblast Phenotype in Pulmonary Interstitial Glycogenosis. Am. J. Respir. Crit. Care Med. 2016, 193, 694–696. [Google Scholar] [CrossRef] [PubMed]
- Galambos, C.; Wartchow, E.; Weinman, J.P.; Abman, S.H. Pulmonary interstitial glycogenosis cells express mesenchymal stem cell markers. Eur. Respir. J. 2020, 56, 2000853. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, G.H.; Young, L.R. Pulmonary interstitial glycogenosis: Words of caution. Pediatr. Radiol. 2010, 40, 1471–1475. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, G.H.; Young, L.R. Histologic resolution of pulmonary interstitial glycogenosis. Pediatr. Dev. Pathol. Off. J. Soc. Pediatr. Pathol. Paediatr. Pathol. Soc. 2009, 12, 475–480. [Google Scholar] [CrossRef]
- Sardón, O.; Torrent-Vernetta, A.; Rovira-Amigo, S.; Dishop, M.K.; Ferreres, J.C.; Navarro, A.; Corcuera, P.; Korta-Murua, J.; Peña, P.G.; Pérez-Belmonte, E.; et al. Isolated pulmonary interstitial glycogenosis associated with alveolar growth abnormalities: A long-term follow-up study. Pediatr. Pulmonol. 2019, 54, 837–846. [Google Scholar] [CrossRef]
- Pulvirenti, G.; Parisi, G.F.; Giallongo, A.; Papale, M.; Manti, S.; Savasta, S.; Licari, A.; Marseglia, G.L.; Leonardi, S. Lower Airway Microbiota. Front. Pediatr. 2019, 7, 393. [Google Scholar] [CrossRef]
- Manti, S.; Parisi, G.F.; Giacchi, V.; Sciacca, P.; Tardino, L.; Cuppari, C.; Chikermane, A.; Leonardi, S. Pilot study shows right ventricular diastolic function impairment in young children with obstructive respiratory disease. Acta Paediatr. 2019, 108, 740–744. [Google Scholar] [CrossRef]
- Marseglia, G.L.; Manti, S.; Chiappini, E.; Brambilla, I.; Caffarelli, C.; Calvani, M.; Cardinale, F.; Cravidi, C.; Duse, M.; Martelli, A.; et al. Chronic cough in childhood: A systematic review for practical guidance by the Italian Society of Pediatric Allergy and Immunology. Allergol. Immunopathol. 2021, 49, 133–154. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Presti, S.; Parisi, G.F.; Papale, M.; Gitto, E.; Manti, S.; Leonardi, S. Interstitial Lung Disease in Children: “Specific Conditions of Undefined Etiology” Becoming Clearer. Children 2022, 9, 1744. https://doi.org/10.3390/children9111744
Presti S, Parisi GF, Papale M, Gitto E, Manti S, Leonardi S. Interstitial Lung Disease in Children: “Specific Conditions of Undefined Etiology” Becoming Clearer. Children. 2022; 9(11):1744. https://doi.org/10.3390/children9111744
Chicago/Turabian StylePresti, Santiago, Giuseppe Fabio Parisi, Maria Papale, Eloisa Gitto, Sara Manti, and Salvatore Leonardi. 2022. "Interstitial Lung Disease in Children: “Specific Conditions of Undefined Etiology” Becoming Clearer" Children 9, no. 11: 1744. https://doi.org/10.3390/children9111744
APA StylePresti, S., Parisi, G. F., Papale, M., Gitto, E., Manti, S., & Leonardi, S. (2022). Interstitial Lung Disease in Children: “Specific Conditions of Undefined Etiology” Becoming Clearer. Children, 9(11), 1744. https://doi.org/10.3390/children9111744







