Reducing Birth Defects by Decreasing the Prevalence of Maternal Chronic Diseases—Evaluated by Linked National Registration Dataset
Abstract
1. Introduction
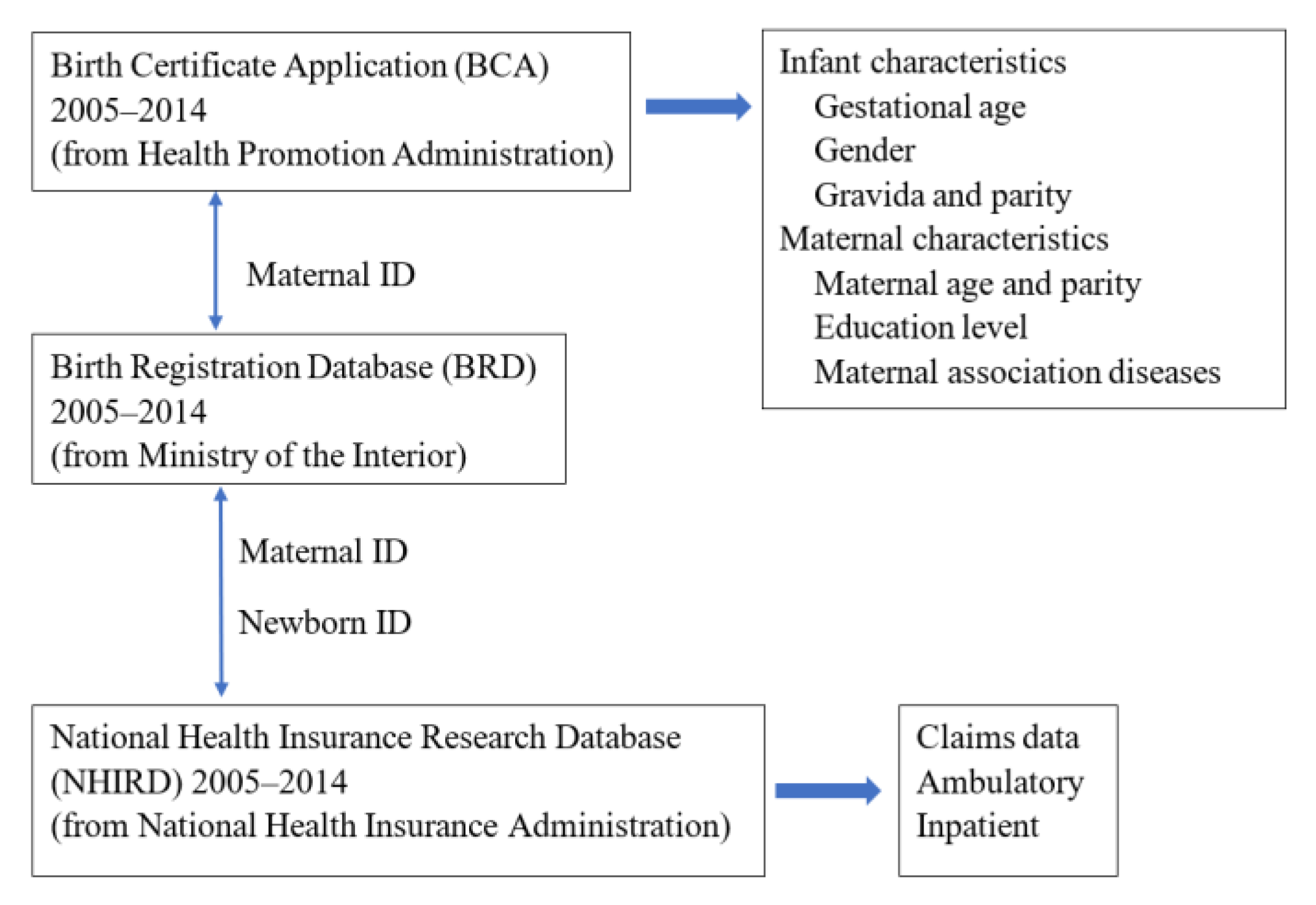
2. Materials and Methods
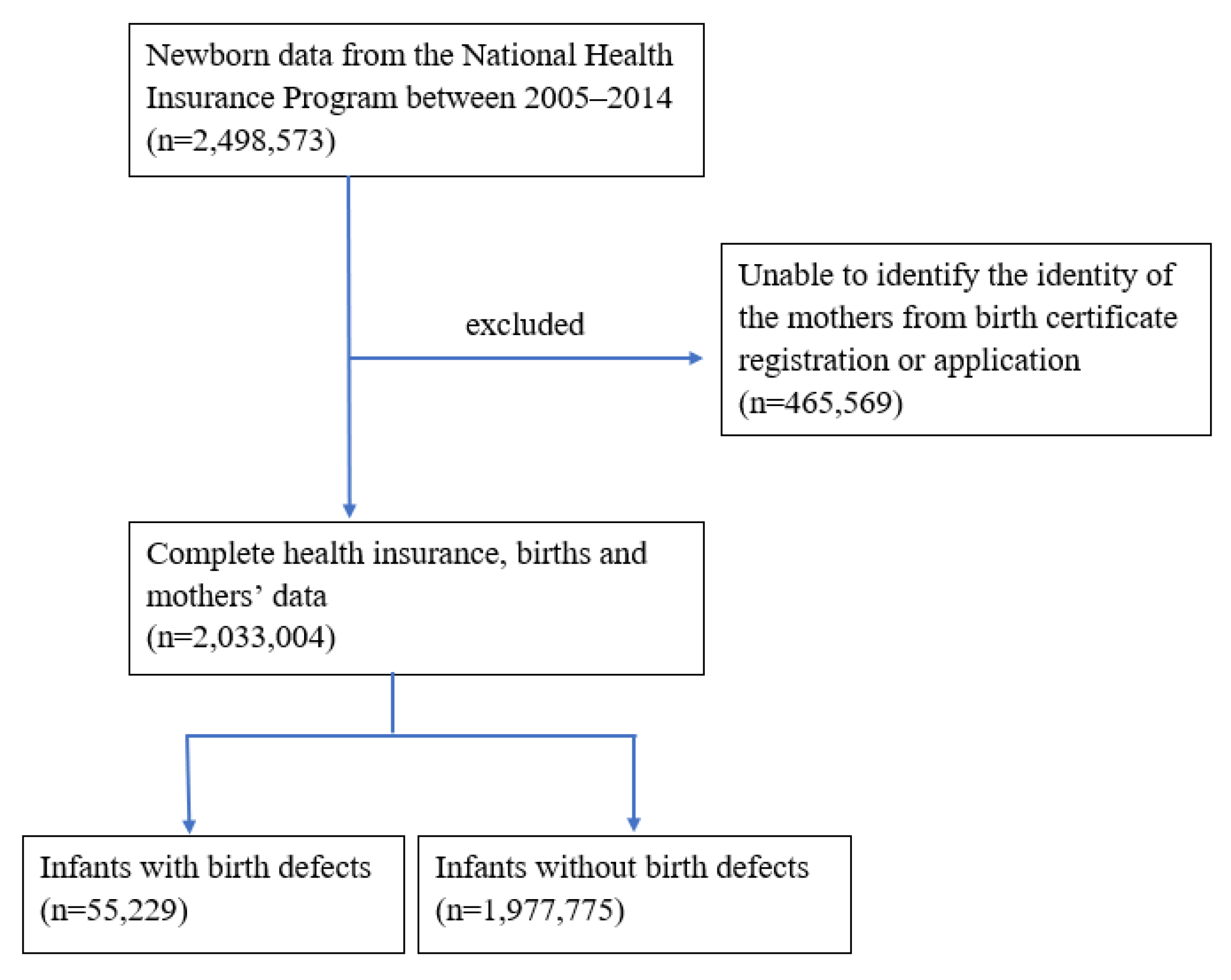
2.1. Study Population
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Congenital Anomalies. 2019. Available online: http://www.who.int/en/news-room/fact-sheets/detail/congenital-anomalies (accessed on 2 July 2019).
- Chen, B.-Y.; Hwang, B.-F.; Guo, Y.-L. Epidemiology of Congenital Anomalies in a Population-based Birth Registry in Taiwan, 2002. J. Formos. Med. Assoc. 2009, 108, 460–468. [Google Scholar] [CrossRef][Green Version]
- Ko, H.S.; Kim, D.J.; Chung, Y.; Wie, J.H.; Choi, S.K.; Park, I.Y.; Park, Y.G.; Shin, J.C. A national cohort study evaluating infant and fetal mortality caused by birth defects in Korea. BMJ Open 2017, 7, e017963. [Google Scholar] [CrossRef]
- Canfield, M.A.; Honein, M.A.; Yuskiv, N.; Xing, J.; Mai, C.T.; Collins, J.S.; Devine, O.; Petrini, J.; Ramadhani, T.A.; Hobbs, C.A.; et al. National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999–2001. Birth Defects Res. Part A Clin. Mol. Teratol. 2006, 76, 747–756. [Google Scholar] [CrossRef]
- Vatankhah, S.; Jalilvand, M.; Sarkhosh, S.; Azarmi, M.; Mohseni, M. Prevalence of Congenital Anomalies in Iran: A Review Article. Iran. J. Public Health 2017, 46, 733–743. [Google Scholar]
- Xie, D.; Yang, T.; Liu, Z.; Wang, H. Epidemiology of Birth Defects Based on a Birth Defect Surveillance System from 2005 to 2014 in Hunan Province, China. PLoS ONE 2016, 11, e0147280. [Google Scholar] [CrossRef] [PubMed]
- Louis, A.M.; Kim, K.; Browne, M.L.; Liu, G.; Liberman, R.F.; Nembhard, W.N.; Confield, M.A.; Copeland, G.; Fornoff, J.; Kirby, R.; et al. Prevalence trends of selected major defects: A multiple-state population-based retrospective study, United State, 1999 to 2007. Birth Defects Res. 2017, 109, 1442–1450. [Google Scholar]
- Tan, K.H.; Tan, T.Y.; Tan, J.; Tan, I.; Chew, S.K.; Yeo, G.S.H. Birth defects in Singapore: 1994–2000. Singap. Med. J. 2005, 46, 545–552. [Google Scholar]
- Boyd, P.A.; Tonks, A.M.; Rankin, J.; Rounding, C.; Wellesley, D.; Draper, E.S.; The Binocar Working Group. Monitoring the Prenatal Detection of Structural Fetal Congenital Anomalies in England and Wales: Register-based Study. J. Med. Screen. 2011, 18, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, A.A.; Brenn, T.; Odland, J.; Nieboer, E.; Krettek, A.; Anda, E.E. Under-reporting of major birth defects in Northwest Russia: A registry-based study. Int. J. Circumpolar Health 2017, 76, 1366785. [Google Scholar] [CrossRef]
- Report EUROCAT Annual Surveillance. 2012. Available online: https://eu-rd-platform.jrc.ec.europa.eu/sites/default/files/Stat-Mon-Report-2012.pdf (accessed on 10 July 2017).
- Chen, L.-J.; Chiou, J.-Y.; Huang, J.-Y.; Su, P.-H.; Chen, J.-Y. Birth defects in Taiwan: A 10-year nationwide population-based, cohort study. J. Formos. Med. Assoc. 2019, 119, 553–559. [Google Scholar] [CrossRef]
- Loane, M.; Dolk, H.; Morris, J.K.; A EUROCAT Working Group. National age-specific risk of non-chromosomal anomalies. BJOG 2009, 116, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, G.; Gualdi, S.; Bower, C.; Halliday, J.; Jonsson, B.; Myrelid, A.; Mastroiacovo, P.; Amar, E.; Bakker, M.K.; Correa, A.; et al. International trends of Down’s syndrome 1993–2004: Births in relation to maternal age and terminations of pregnancies. Birth Defects Res A Clin Mol Teratol. 2010, 88, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Dolk, H.; Vrijheid, M. The impact of environmental pollution on congenital anomalies. Br. Med. Bull. 2003, 68, 25–45. [Google Scholar] [CrossRef]
- Lisi, A.; Botto, L.D.; Robert-Gnansia, E.; Castilla, E.E.; Bakker, M.K.; Bianca, S.; Cocchi, G.; de Vigan, C.; Dutra, M.D.G.; Horacek, J.; et al. Surveillance of adverse fetal effects of medications (SAFE-Med): Findings from the International Clearinghouse of Birth Defects Surveillance and Research. Reprod. Toxicol. 2010, 29, 433–442. [Google Scholar] [CrossRef]
- Garne, E.; Hansen, A.V.; Morris, J.; Zaupper, L.; Addor, M.C.; Barisic, I.; Gatt, M.; Lelong, N.; Klungsøyr, K.; O’Mahony, M.; et al. Use of asthma medi-cation during pregnancy and risk of specific congenital anomalies: A European case-malformed control study. J. Allergy Clin. Immunol. 2015, 136, 1496–1502. [Google Scholar] [CrossRef]
- Crider, K.S.; Cleves, M.A.; Reefhuis, J.; Berry, R.J.; Hobbs, C.A.; Hu, D.J. Antibacterial medication use during pregnancy and risk of birth defects: National birth defects prevention study. Arch Pediatr. Adolesc. Med. 2009, 163, 978–985. [Google Scholar] [CrossRef]
- Chen, C.-H.; Xirasagar, S.; Lin, C.-C.; Wang, L.-H.; Kou, Y.; Lin, H.-C. Risk of adverse perinatal outcomes with antithyroid treatment during pregnancy: A nationwide population-based study. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Clementi, M.; Di Gianantonio, E.; Cassina, M.; Leoncini, E.; Botto, L.D.; Mastroiacovo, P.; The SAFE-Med Study Group. Treatment of hyperthyroid-ism in pregnancy and birth defects. J. Clin. Endocrinol. Metab. 2010, 95, E337–E341. [Google Scholar] [CrossRef] [PubMed]
- Wren, C.; Birrell, G.; Hawthorne, G. Cardiovascular malformations in infants of diabetic mothers. Heart 2003, 89, 1217–1220. [Google Scholar] [CrossRef]
- Correa, A.; Gilboa, S.M.; Besser, L.M.; Botto, L.D.; Moore, C.A.; Hobbs, C.A.; Cleves, M.A.; Riehle-Colarusso, T.J.; Waller, D.K.; Reece, E.A.; et al. Diabe-tes mellitus and birth defects. Am. J. Obstet. Gynecol. 2008, 199, 237.e1–237.e9. [Google Scholar] [CrossRef]
- Aberg, A.; Westbom, L.; Kallen, B. Congenital malformations among infants whose mothers had gestational diabetes or preexisting diabetes. Early Hum. Dev. 2001, 61, 85–95. [Google Scholar] [CrossRef]
- Becerra, J.E.; Khoury, M.J.; Cordero, J.F.; Erickson, J.D. Diabetes mellitus during pregnancy and the risks for specific birth defects: A population-based case-control study. Pediatrics 1990, 85, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cummings, E.A.; O’connell, C.; Jangaard, K. Fetal and Neonatal Outcomes of Diabetic Pregnancies. Obstet. Gynecol. 2006, 108, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.L. Malformations in infants of diabetic mothers. Birth Defects Res. A Clin. Mol. Teratol. 2010, 88, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Nederstigt, C.; Uitbeijerse, B.S.; Janssen, L.G.M.; Corssmit, E.P.M.; de Koning, E.J.P. Associated auto-immune disease in type 1 diabetes patients: A systematic review and metaeanalysis. Eur. J. Endocrinol. 2019, 180, 135–144. [Google Scholar] [CrossRef]
- Chou, H.-H.; Chiou, M.-J.; Liang, F.-W.; Chen, L.-H.; Lu, T.-H.; Li, C.-Y. Association of maternal chronic disease with risk of congenital heart disease in offspring. Can. Med. Assoc. J. 2016, 188, E438–E446. [Google Scholar] [CrossRef]
- Feldkamp, M.L.; Reefhuis, J.; Kucik, J.; Krikov, S.; Wilson, A.; Moore, C.A.; Carey, J.C.; Botto, L.D. Case-control study of self reported genitourinary infections and risk of gastroschisis: Findings from the national birth defects prevention study, 1997–2003. BMJ 2008, 336, 1420–1423. [Google Scholar] [CrossRef]
- Stothard, K.J.; Tennant, P.W.G.; Bell, R.; Rankin, J. Maternal overweight and obesity and the risk of congenital anomalies: A systematic review and meta-analysis. JAMA 2009, 301, 636–650. [Google Scholar] [CrossRef]
- Hsing, A.W.; Ioannidis, J.P.A. Lessons from the Taiwan National Health Insurance Research Database. JAMA Intern Med. 2015, 175, 1527–1529. [Google Scholar] [CrossRef]
- Hsieh, C.-Y.; Su, C.-C.; Shao, S.-C.; Sung, S.-F.; Lin, S.-J.; Yang, Y.-H.K.; Lai, E.C.C. Taiwan’s National Health Insurance Research Database: Past and Future. Clin. Epidemiol. 2019, 11, 349–358. [Google Scholar] [CrossRef]
- National Developmental Council Population Projections for R.O.C (Taiwan). Available online: https://pop-proj.ndc.gov.tw//main_en/index.aspx (accessed on 12 September 2022).
- Morris, J.K.; Springett, A.L.; Greenlees, R.; Loane, M.; Addor, M.C.; Arriola, L.; Barisic, I.; Bergman, J.E.H.; Csaky-Szunyogh, M.; Dias, C.; et al. Trends in congenital anomalies in Europe from 1980 to 2012. PLoS ONE 2018, 13, e0194986. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-K.; Lamichhane, D.K.; Kim, H.-C.; Leem, J.-H. Trends in the Prevalences of Selected Birth Defects in Korea (2008–2014). Int. J. Environ. Res. Public Health 2018, 15, 923. [Google Scholar] [CrossRef] [PubMed]
- Mai, C.T.; Isenburg, J.; Langlois, P.H.; Alverson, C.J.; Gilboa, S.M.; Rickard, R.; Canfield, M.A.; Anjohrin, S.B.; Lupo, P.J.; Jackson, D.R.; et al. Population-based birth defects data in the United States, 2008 to 2012: Presentation of state-specific data and descriptive brief on variability of prevalence. Birth Defects Res. Part A Clin. Mol. Teratol. 2015, 103, 972–993. [Google Scholar] [CrossRef] [PubMed]
| Maternal Diseases | No BDs | BDs | Prevalence Rate (per 10,000 Births) | RR | p Value |
|---|---|---|---|---|---|
| Cardiovascular diseases | 4208 (0.21%) | 436 (0.79%) | 938.85 | 3.38 | <0.0001 |
| Hypertension | 42,648 (2.16%) | 2156 (3.90%) | 481.21 | 1.75 | <0.0001 |
| Renal diseases | 6859 (0.35%) | 302 (0.55%) | 421.73 | 1.51 | <0.0001 |
| Genitourinary infections | 124,044 (6.27%) | 4147 (7.51%) | 323.50 | 1.17 | <0.0001 |
| Anemia | 110,210 (5.57%) | 3416 (6.19%) | 300.64 | 1.08 | <0.0001 |
| Neurotic and psychotic disorders | 54,309 (2.75%) | 1689 (3.06%) | 301.62 | 1.08 | 0.0013 |
| Alcohol-related conditions | 1013 (0.05%) | 39 (0.07%) | 370.72 | 1.33 | 0.0767 |
| Drug abuse and dependence | 5091 (0.26%) | 160 (0.29%) | 304.70 | 1.09 | 0.2692 |
| DM | |||||
| No DM | 1,719,174 (86.92%) | 47,635 (86.25%) | 269.61 | Reference | |
| GDM | 237,122 (11.99%) | 6866 (12.43%) | 281.41 | 1.04 | 0.0009 |
| DM (type 1 or type 2) | 21,479 (1.09%) | 728 (1.32%) | 327.82 | 1.22 | <0.0001 |
| Maternal Disease | Number of Cases | Prevalence Rate | Relative Risk | PAR% |
|---|---|---|---|---|
| Hypertension | 44,804 | 2.20% | 1.75 | 1.63% |
| Cardiovascular disease | 4644 | 0.23% | 3.38 | 0.55% |
| Renal disease | 7161 | 0.35% | 1.51 | 0.18% |
| Genitourinary infection | 128,191 | 6.31% | 1.17 | 1.06% |
| Anemia | 113,626 | 5.59% | 1.08 | 0.45% |
| Neurotic and psychotic disorders | 55,998 | 2.75% | 1.08 | 0.22% |
| GDM | 243,988 | 12.00% | 1.04 | 0.48% |
| DM (type 1 or type 2) | 22,207 | 1.09% | 1.22 | 0.24% |
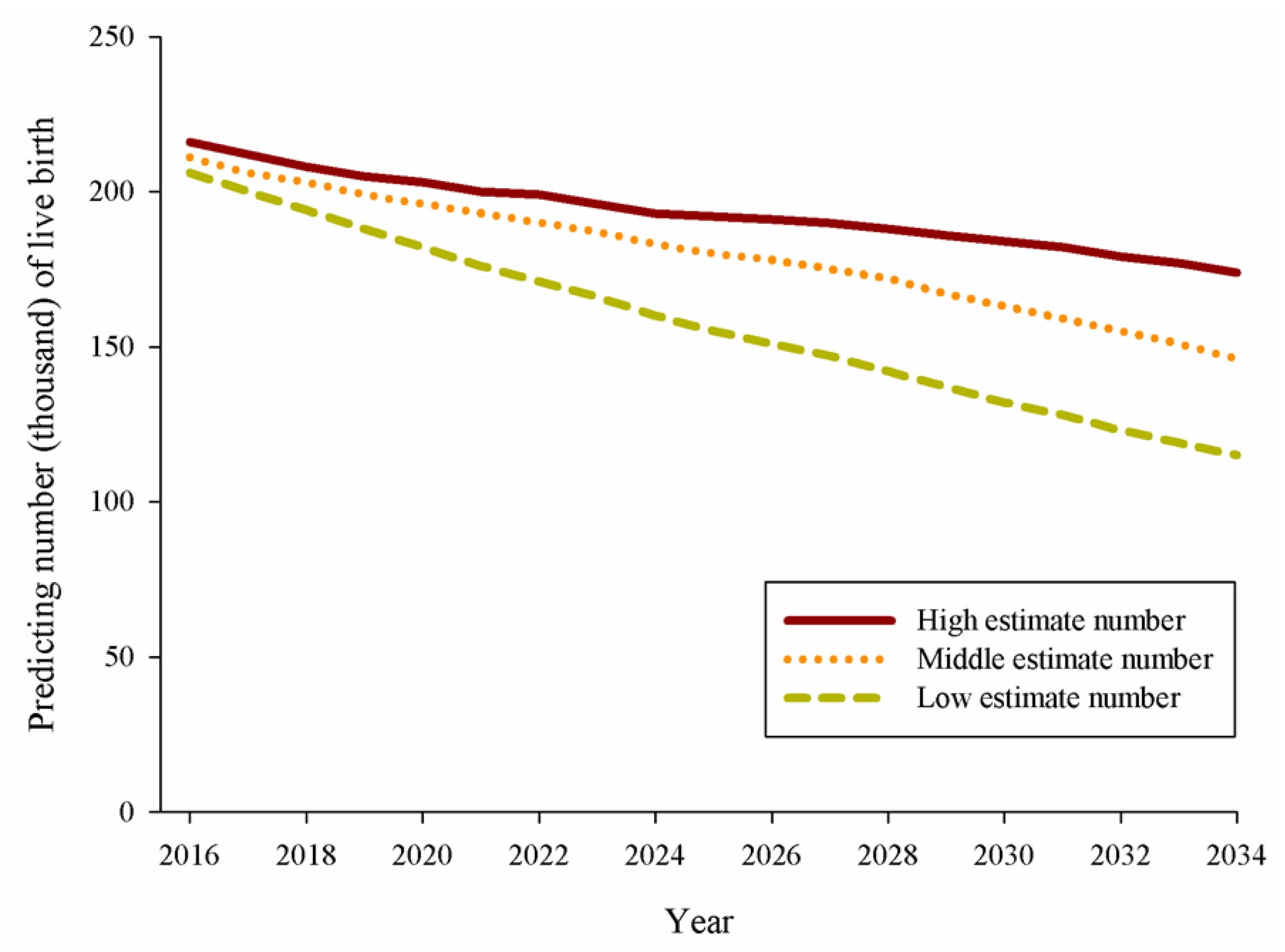
| Percentage Change of the Prevalence of Maternal Disease in 2034 † | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| −1% | −5% | −10% | |||||||
| Prevalence of Maternal Disease | HE | ME | LE | HE | ME | LE | HE | ME | LE |
| Hypertension | 73 | 60 | 48 | 363 | 302 | 239 | 725 | 604 | 479 |
| Cardiovascular disease | 25 | 20 | 16 | 123 | 102 | 81 | 245 | 204 | 162 |
| Renal diseases | 8 | 6 | 5 | 40 | 33 | 26 | 80 | 67 | 53 |
| Genitourinary infection | 47 | 39 | 31 | 236 | 196 | 156 | 472 | 393 | 311 |
| Anemia | 20 | 16 | 13 | 100 | 83 | 66 | 201 | 167 | 132 |
| Neurotic and psychotic disorders | 10 | 8 | 6 | 49 | 41 | 32 | 98 | 81 | 64 |
| GDM | 22 | 18 | 14 | 107 | 89 | 70 | 214 | 178 | 141 |
| Pregestational (Type 1 or type 2) DM | 11 | 9 | 7 | 54 | 44 | 35 | 107 | 89 | 70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-J.; Chen, P.-J.; Huang, J.-Y.; Yang, S.-F.; Chen, J.-Y. Reducing Birth Defects by Decreasing the Prevalence of Maternal Chronic Diseases—Evaluated by Linked National Registration Dataset. Children 2022, 9, 1793. https://doi.org/10.3390/children9121793
Chen L-J, Chen P-J, Huang J-Y, Yang S-F, Chen J-Y. Reducing Birth Defects by Decreasing the Prevalence of Maternal Chronic Diseases—Evaluated by Linked National Registration Dataset. Children. 2022; 9(12):1793. https://doi.org/10.3390/children9121793
Chicago/Turabian StyleChen, Lih-Ju, Ping-Ju Chen, Jing-Yang Huang, Shun-Fa Yang, and Jia-Yuh Chen. 2022. "Reducing Birth Defects by Decreasing the Prevalence of Maternal Chronic Diseases—Evaluated by Linked National Registration Dataset" Children 9, no. 12: 1793. https://doi.org/10.3390/children9121793
APA StyleChen, L.-J., Chen, P.-J., Huang, J.-Y., Yang, S.-F., & Chen, J.-Y. (2022). Reducing Birth Defects by Decreasing the Prevalence of Maternal Chronic Diseases—Evaluated by Linked National Registration Dataset. Children, 9(12), 1793. https://doi.org/10.3390/children9121793







