Neurological Involvement in Children with COVID-19 and MIS-C: A Retrospective Study Conducted for More than Two Years in a Pediatric Hospital
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Cases Definition
2.2. Statistical Analysis
3. Results
3.1. Epidemiology
3.2. Clinical Manifestations
3.2.1. COVID-19 Group
3.2.2. MIS-C Group
3.2.3. COVID-19 and MIS-C Comparisons
3.3. Laboratory Tests
3.4. Instrumental Investigations
3.5. Management
3.5.1. COVID-19 Group
3.5.2. MIS-C Group
3.6. Course and Outcome
3.7. Specific Neurological Disorders
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Mo, X.; Hu, Y.; Qi, X.; Jiang, F.; Jiang, Z.; Tong, S. Epidemiology of COVID-19 Among Children in China. Pediatrics 2020, 145, e20200702. [Google Scholar] [CrossRef] [Green Version]
- Ludvigsson, J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020, 109, 1088–1095. [Google Scholar] [CrossRef]
- Siddique, R.; Khan, S.; Shabana; Li, M.; Xue, M.; Ghanim, K.; Kaimkhani, Z.A.; Mahboob, S. Neurological complications of COVID-19 in children and the associated immunological responses. J. King Saud Univ. Sci. 2022, 34, 101884. [Google Scholar] [CrossRef]
- Lin, J.E.; Asfour, A.; Sewell, T.B.; Hooe, B.; Pryce, P.; Earley, C.; Shen, M.Y.; Kerner-Rossi, M.; Thakur, K.T.; Vargas, W.S.; et al. Neurological issues in children with COVID-19. Neurosci. Lett. 2020, 743, 135567. [Google Scholar] [CrossRef]
- Oualha, M.; Bendavid, M.; Berteloot, L.; Corsia, A.; Lesage, F.; Vedrenne, M.; Salvador, E.; Grimaud, M.; Chareyre, J.; de Marcellus, C.; et al. Severe and fatal forms of COVID-19 in children. Archives de Pédiatrie 2020, 27, 235–238. [Google Scholar] [CrossRef]
- Lai, C.-C.; Ko, W.-C.; Lee, P.-I.; Jean, S.-S.; Hsueh, P.-R. Extra-respiratory manifestations of COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106024. [Google Scholar] [CrossRef]
- Kuttiatt, V.; Abraham, P.; Menon, R.; Vaidya, P.; Rahi, M. Coronavirus disease 2019 in children: Clinical epidemiological implications. Indian J. Med Res. 2020, 152, 21–40. [Google Scholar] [CrossRef]
- Montalvan, V.; Lee, J.; Bueso, T.; De Toledo, J.; Rivas, K. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin. Neurol. Neurosurg. 2020, 194, 105921. [Google Scholar] [CrossRef]
- Al-Ramadan, A.; Rabab’H, O.; Shah, J.; Gharaibeh, A. Acute and Post-Acute Neurological Complications of COVID-19. Neurol. Int. 2021, 13, 102–119. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Asadi-Pooya, A.A.; Simani, L. Central nervous system manifestations of COVID-19: A systematic review. J. Neurol. Sci. 2020, 413, 116832. [Google Scholar] [CrossRef]
- Chou, S.H.Y.; Beghi, E.; Helbok, R.; Moro, E.; Sampson, J.; Altamirano, V.; Mainali, S.; Bassetti, C.; Suarez, J.I.; McNett, M.; et al. Global incidence of neurological manifestations among patients hospitalized with COVID-19-A report for the GCSNeuroCOVID consortium and the ENERGY consortium. JAMA Netw. Open 2021, 4, e2112131. [Google Scholar] [CrossRef]
- Varatharaj, A.; Thomas, N.; Ellul, M.A.; Davies, N.W.S.; Pollak, T.A.; Tenorio, E.L.; Sultan, M.; Easton, A.; Breen, G.; Zandi, M.; et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet Psychiatry 2020, 7, 875–882. [Google Scholar] [CrossRef]
- Schou, T.M.; Joca, S.; Wegener, G.; Bay-Richter, C. Psychiatric and neuropsychiatric sequelae of COVID-19—A systematic review. Brain Behav. Immun. 2021, 97, 328–348. [Google Scholar] [CrossRef]
- Ray, S.T.; Abdel-Mannan, O.; Sa, M.; Fuller, C.; Wood, G.K.; Pysden, K.; Yoong, M.; McCullagh, H.; Scott, D.; McMahon, M.; et al. Neurological manifestations of SARS-CoV-2 infection in hospitalised children and adolescents in the UK: A prospective national cohort study. Lancet Child Adolesc. Health 2021, 5, 631–641. [Google Scholar] [CrossRef]
- Fink, E.L.; Robertson, C.L.; Wainwright, M.S.; Roa, J.D.; Lovett, M.E.; Stulce, C.; Yacoub, M.; Potera, R.M.; Zivick, E.; Holloway, A.; et al. Prevalence and Risk Factors of Neurologic Manifestations in Hospitalized Children Diagnosed with Acute SARS-CoV-2 or MIS-C. Pediatr. Neurol. 2021, 128, 33–44. [Google Scholar] [CrossRef]
- Riva, A.; Piccolo, G.; Balletti, F.; Binelli, M.; Brolatti, N.; Verrotti, A.; Amadori, E.; Spalice, A.; Giacomini, T.; Mancardi, M.M.; et al. Acute Neurological Presentation in Children with SARS-CoV-2 Infection. Front. Pediatr. 2022, 10, 909849. [Google Scholar] [CrossRef]
- CDC Health Alert Network. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19). Available online: https://emergency.cdc.gov/han/2020/han00432.asp (accessed on 29 August 2022).
- Pouletty, M.; Borocco, C.; Ouldali, N.; Caseris, M.; Basmaci, R.; Lachaume, N.; Bensaid, P.; Pichard, S.; Kouider, H.; Morelle, G.; et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): A multicentre cohort. Ann. Rheum. Dis. 2020, 79, 999–1006. [Google Scholar] [CrossRef]
- Chiotos, K.; Bassiri, H.; Behrens, E.M.; Blatz, A.M.; Chang, J.; Diorio, C.; Fitzgerald, J.C.; Topjian, A.; John, A.R.O. Multisystem Inflammatory Syndrome in Children During the Coronavirus 2019 Pandemic: A Case Series. J. Pediatr. Infect. Dis. Soc. 2020, 9, 393–398. [Google Scholar] [CrossRef]
- Dufort, E.M.; Koumans, E.H.; Chow, E.; Rosenthal, E.M.; Muse, A.; Rowlands, J.; Barranco, M.A.; Maxted, A.M.; Rosenberg, E.S.; Easton, D.; et al. New York State and CDC Multisystem Inflammatory Syndrome in Children Investigation Team. Multisystem Inflammatory Syndrome in Children in New York State. N. Engl. J. Med. 2020, 383, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Verdoni, L.; Mazza, A.; Gervasoni, A.; Martelli, L.; Ruggeri, M.; Ciuffreda, M.; Bonanomi, E.; D’Antiga, L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: An observational cohort study. Lancet 2020, 395, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mannan, O.; Eyre, M.; Löbel, U.; Bamford, A.; Eltze, C.; Hameed, B.; Hemingway, C.; Hacohen, Y. Neurologic and Radiographic Findings Associated with COVID-19 Infection in Children. JAMA Neurol. 2020, 77, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, S.; Basso, E.; Lavatelli, R.; Previtali, R.; Parenti, L.; Fiori, L.; Dilillo, D.; Zuccotti, G.V.; Veggiotti, P.; Bova, S.M. Acute encephalitis in pediatric multisystem inflammatory syndrome associated with COVID-19. Eur. J. Paediatr. Neurol. 2021, 34, 84–90. [Google Scholar] [CrossRef]
- LaRovere, K.L.; Riggs, B.J.; Poussaint, T.Y.; Young, C.C.; Newhams, M.M.; Maamari, M.; Walker, T.C.; Singh, A.R.; Dapul, H.; Hobbs, C.V.; et al. Neurologic Involvement in Children and Adolescents Hospitalized in the United States for COVID-19 or Multisystem Inflammatory Syndrome. JAMA Neurol. 2021, 78, 536. [Google Scholar] [CrossRef]
- Sánchez-Morales, A.E.; Urrutia-Osorio, M.; Camacho-Mendoza, E.; Rosales-Pedraza, G.; Dávila-Maldonado, L.; González-Duarte, A.; Herrera-Mora, P.; Ruiz-García, M. Neurological manifestations temporally associated with SARS-CoV-2 infection in pediatric patients in Mexico. Child’s Nerv. Syst. 2021, 37, 2305–2312. [Google Scholar] [CrossRef]
- Chen, T.-H. Neurological involvement associated with COVID-19 infection in children. J. Neurol. Sci. 2020, 418, 117096. [Google Scholar] [CrossRef]
- Bartley, C.M.; Johns, C.; Ngo, T.T.; Dandekar, R.; Loudermilk, R.L.; Alvarenga, B.D.; Hawes, I.A.; Zamecnik, C.R.; Zorn, K.C.; Alexander, J.R.; et al. Anti–SARS-CoV-2 and Autoantibody Profiles in the Cerebrospinal Fluid of 3 Teenaged Patients with COVID-19 and Subacute Neuropsychiatric Symptoms. JAMA Neurol. 2021, 78, 1503. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J.; Zhang, W.; Wang, Y.; Bao, S.; Li, Y.; et al. SARS-CoV-2 Infection in Children. N. Engl. J. Med. 2020, 382, 1663–1665. [Google Scholar] [CrossRef] [Green Version]
- Iadecola, C.; Anrather, J.; Kamel, H. Effects of COVID-19 on the Nervous System. Cell 2020, 183, 16–27.e1. [Google Scholar] [CrossRef]
- Duong, L.; Xu, P.; Liu, A. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in Downtown Los Angeles, early April 2020. Brain Behav. Immun. 2020, 87, 33. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Jiang, D.; Huang, J.T. SARS-CoV-2 Detected in Cerebrospinal Fluid by PCR in a Case of COVID-19 Encephalitis. Brain, Behav. Immun. 2020, 87, 149. [Google Scholar] [CrossRef] [PubMed]
- Araújo, N.M.; Ferreira, L.C.; Dantas, D.P.; Silva, D.S.; dos Santos, C.A.; Cipolotti, R.; Martins-Filho, P.R. First Report of SARS-CoV-2 Detection in Cerebrospinal Fluid in a Child with Guillain-Barré Syndrome. Pediatr. Infect. Dis. J. 2021, 40, e274–e276. [Google Scholar] [CrossRef] [PubMed]
- Zamani, R.; Pouremamali, R.; Rezaei, N. Central neuroinflammation in COVID-19: A systematic review of 182 cases with en-cephalitis, acute disseminated encephalomyelitis, and necrotizing encephalopathies. Rev. Neurosci. 2021, 33, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Paniz-Mondolfi, A.; Bryce, C.; Grimes, Z.; Gordon, R.E.; Reidy, J.; Lednicky, J.; Sordillo, E.M.; Fowkes, M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J. Med Virol. 2020, 92, 699–702. [Google Scholar] [CrossRef] [Green Version]
- Lewis, A.; Frontera, J.; Placantonakis, D.G.; Lighter, J.; Galetta, S.; Balcer, L.; Melmed, K.R. Cerebrospinal fluid in COVID-19: A systematic review of the literature. J. Neurol. Sci. 2021, 421, 117316. [Google Scholar] [CrossRef]
- Tandon, M.; Kataria, S.; Patel, J.; Mehta, T.R.; Daimee, M.; Patel, V.; Prasad, A.; Chowdhary, A.A.; Jaiswal, S.; Sriwastava, S. A Comprehensive Systematic Review of CSF analysis that defines Neurological Manifestations of COVID-19. Int. J. Infect. Dis. 2021, 104, 390–397. [Google Scholar] [CrossRef]
- Khan, S.; Siddique, R.; Hao, X.; Lin, Y.; Liu, Y.; Wang, X.; Hua, L.; Nabi, G. The COVID-19 infection in children and its association with the immune system, prenatal stress, and neurological complications. Int. J. Biol. Sci. 2022, 18, 707–716. [Google Scholar] [CrossRef]
- Panda, P.K.; Sharawat, I.K.; Panda, P.; Natarajan, V.; Bhakat, R.; Dawman, L. Neurological Complications of SARS-CoV-2 Infection in Children: A Systematic Review and Meta-Analysis. J. Trop. Pediatr. 2020, 67, fmaa070. [Google Scholar] [CrossRef]
- Kurd, M.; Hashavya, S.; Benenson, S.; Gilboa, T. Seizures as the main presenting manifestation of acute SARS-CoV-2 infection in children. Seizure 2021, 92, 89–93. [Google Scholar] [CrossRef]
- Ludvigsson, J.F. Convulsions in children with COVID-19 during the Omicron wave. Acta Paediatr. 2022, 111, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.epicentro.iss.it/coronavirus/sars-cov-2-monitoraggio-varianti-rapporti-periodici (accessed on 4 September 2022).
- Abel, D.; Shen, M.Y.; Abid, Z.; Hennigan, C.; Boneparth, A.; Miller, E.H.; Uhlemann, A.-C.; McBrian, D.K.; Thakur, K.; Silver, W.; et al. Encephalopathy and bilateral thalamic lesions in a child with MIS-C associated with COVID-19. Neurology 2020, 95, 745–748. [Google Scholar] [CrossRef]
- Misra, S.; Kolappa, K.; Prasad, M.; Radhakrishnan, D.; Thakur, K.T.; Solomon, T.; Michael, B.D.; Winkler, A.S.; Beghi, E.; Guekht, A.; et al. Frequency of Neurologic Manifestations in COVID-19. Neurology 2021, 97, e2269–e2281. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Roy, D.; Sinha, K.; Parveen, S.; Sharma, G.; Joshi, G. Impact of COVID-19 and lockdown on mental health of children and adolescents: A narrative review with recommendations. Psychiatry Res. 2020, 293, 113429. [Google Scholar] [CrossRef] [PubMed]
- Beslow, L.A.; Msc, A.B.L.; Fox, C.K.; Kossorotoff, M.; Zambrano, Y.C.Z.; Hernández-Chávez, M.; Hassanein, S.M.A.; Byrne, S.; Lim, M.; Maduaka, N.; et al. Pediatric Ischemic Stroke: An Infrequent Complication of SARS-CoV-2. Ann. Neurol. 2020, 89, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Mac Grory, B.; Schrag, M.; Biousse, V.; Furie, K.L.; Gerhard-Herman, M.; Lavin, P.J.; Sobrin, L.; Tjoumakaris, S.I.; Weyand, C.M.; Yaghi, S. Management of Central Retinal Artery Occlusion: A Scientific Statement from the American Heart Association. Stroke 2021, 52, e282–e294. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Diamond, M.; Anwar, S.; Glaser, A.; Tyagi, P. Unique case of central retinal artery occlusion secondary to COVID-19 disease. IDCases 2020, 21, e00867. [Google Scholar] [CrossRef] [PubMed]
- Montesel, A.; Bucolo, C.; Mouvet, V.; Moret, E.; Eandi, C.M. Case Report: Central Retinal Artery Occlusion in a COVID-19 Patient. Front. Pharmacol. 2020, 11, 588384. [Google Scholar] [CrossRef]
- Kulkarni, M.; Rajesh, R.; Shanmugam, M. Ocular occlusions in two cases of COVID-19. Indian J. Ophthalmol. 2022, 70, 1825. [Google Scholar] [CrossRef]
- Honavar, S.; Sen, M.; Sharma, N.; Sachdev, M. COVID-19 and Eye: A Review of Ophthalmic Manifestations of COVID-19. Indian J. Ophthalmol. 2021, 69, 488–509. [Google Scholar] [CrossRef]
- Aladawi, M.; Elfil, M.; Abu-Esheh, B.; Abu Jazar, D.; Armouti, A.; Bayoumi, A.; Piccione, E. Guillain Barre Syndrome as a Complication of COVID-19: A Systematic Review. Can. J. Neurol. Sci. J. Can. des Sci. Neurol. 2021, 49, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Mussinatto, I.; Benevenuta, C.; Caci, A.; Calvo, M.M.; Impastato, M.; Barra, M.; Genovese, E.; Timeus, F. Possible association between Guillain-Barré syndrome and SARS-CoV-2 infection in children: A case report and literature review. Exp. Ther. Med. 2022, 24, 462. [Google Scholar] [CrossRef] [PubMed]
- Lindan, C.E.; Mankad, K.; Ram, D.; Kociolek, L.K.; Silvera, V.M.; Boddaert, N.; Stivaros, S.M.; Palasis, S.; Akhtar, S.; Alden, D.; et al. Neuroimaging manifestations in children with SARS-CoV-2 infection: A multinational, multicentre collaborative study. Lancet Child Adolesc. Health 2020, 5, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, A.; Tunkel, A.R.; Bloch, K.C.; Lauring, A.S.; Sejvar, J.; Bitnun, A.; Stahl, J.-P.; Mailles, A.; Drebot, M.; Rupprecht, C.E.; et al. Case Definitions, Diagnostic Algorithms, and Priorities in Encephalitis: Consensus Statement of the International Encephalitis Consortium. Clin. Infect. Dis. 2013, 57, 1114–1128. [Google Scholar] [CrossRef] [Green Version]
- Henderson, L.A.; Canna, S.W.; Friedman, K.G.; Gorelik, M.; Lapidus, S.K.; Bassiri, H.; Behrens, E.M.; Kernan, K.F.; Schulert, G.S.; Seo, P.; et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated with SARS–CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 3. Arthritis Rheumatol. 2022, 74, e1–e20. [Google Scholar] [CrossRef]
- Ding, K.; De La Plata, C.M.; Wang, J.Y.; Mumphrey, M.; Moore, C.; Harper, C.; Madden, C.J.; McColl, R.; Whittemore, A.; Devous, M.D.; et al. Cerebral Atrophy after Traumatic White Matter Injury: Correlation with Acute Neuroimaging and Outcome. J. Neurotrauma 2008, 25, 1433–1440. [Google Scholar] [CrossRef]
- Ostrov, S.G.; Quencer, R.M.; Gaylis, N.B.; Altman, R.D. Cerebral atrophy in systemic lupus erythematosus: Steroid- or disease-induced phenomenon? Am. J. Neuroradiol. 1982, 3, 21–23. [Google Scholar]
- Bassal, F.C.; Harwood, M.; Oh, A.; Lundberg, J.N.; Hoffman, J.; Cornejo, P.; Chapple, K.M.; Hughes, J.N.; Narayan, R. Anti-NMDA receptor encephalitis and brain atrophy in children and adults: A quantitative study. Clin. Imaging 2021, 78, 296–300. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Nemati, H.; Shahisavandi, M.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; et al. Long COVID in children and adolescents. World J. Pediatr. 2021, 17, 495–499. [Google Scholar] [CrossRef]
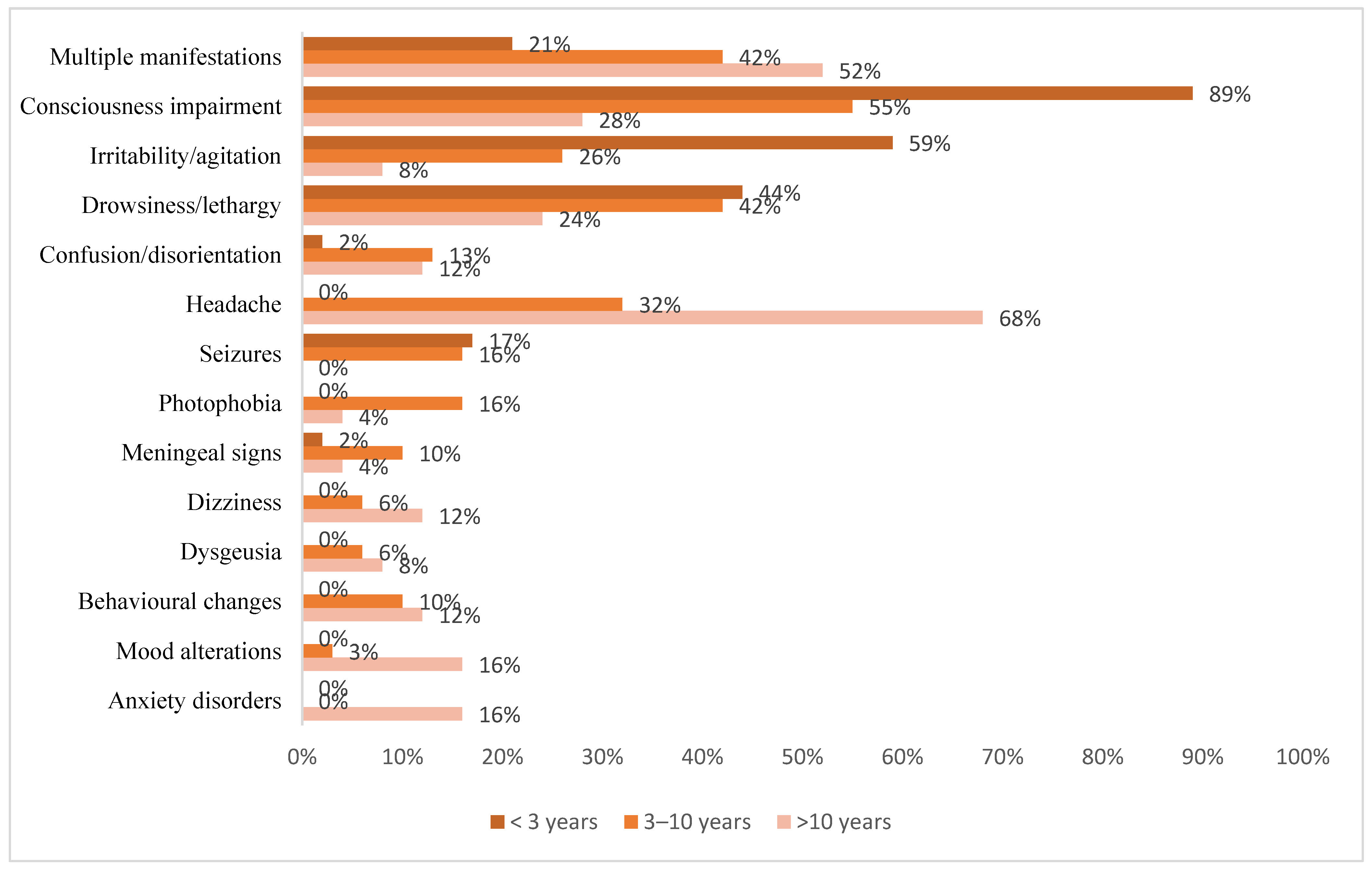



| Epidemiological Data | Total (n = 122) | COVID-19 (n = 95) | MIS-C (n = 27) | p |
|---|---|---|---|---|
| Age at onset, median (IQR)—years | 1.98 (0.34–8.57) | 0.94 (0.21–6.98) | 8.5 (3.68–11.62) | <0.001 |
| Gender: Male, n (%) | 74 (60.7) | 53 (55.8) | 21 (77.8) | 0.039 |
| Ethnicity: Caucasian, n (%) | 101 (82.8) | 81 (85.3) | 20 (74.1) | 0.227 |
| Others, n (%) | 21 (17.2) | 14 (14.7) | 7 (25.9) | |
| Asiatic, n | 5 | 2 | 3 | |
| Hispanic, n | 4 | 4 | 0 | |
| African, n | 3 | 0 | 3 | |
| Pacific Islands, n | 1 | 1 | 0 | |
| Not specified, n | 8 | 7 | 1 | |
| Familiar history #, n (%) | 23 (18.9) | 20 (21.1) | 3 (11.1) | 0.402 |
| Comorbidities, n (%) | 53 (43.4) | 42 (44.2) | 11 (40.7) | 0.748 |
| Neuropsychiatric comorbidities, n (%) | 24 (19.7) | 21 (22.1) | 3 (11.1) | 0.277 |
| Time between onset and hospitalization, median (IQR)—days | 3 (1–5) | 2 (1–5) | 4 (3–6) | <0.001 |
| Neurological Manifestations | Total (n = 122) | COVID-19 (n = 95) | MIS-C (n = 27) | p |
|---|---|---|---|---|
| Multiple manifestations, n (%) | 40 (32.8) | 24 (25.3) | 16 (59.3) | <0.001 |
| Consciousness impairment, n (%) | 83 (68) | 64 (67.4) | 19 (70.4) | 0.822 |
| Irritability/agitation, n (%) | 49 (40.2) | 37 (38.9) | 12 (44.4) | 0.607 |
| Drowsiness/hyporeactivity, n (%) | 48 (39.3) | 32 (33.7) | 16 (59.3) | 0.016 |
| Confusion, n (%) | 8 (6.6) | 3 (3.2) | 5 (18.5) | 0.013 |
| Temporary LOC, n (%) | 2 (1.6) | 2 (2.1) | 0 | 1 |
| Sopor/stupor, n (%) | 3 (2.5) | 0 | 3 (11.1) | 0.01 |
| Headache, n (%) | 27 (22.1) | 18 (18.9) | 9 (33.3) | 0.112 |
| Seizures, n (%) | 16 (13.1) | 16 (16.8) | 0 | 0.021 |
| Behavioural changes, n (%) | 6 (4.9) | 1 (1.1) | 5 (18.5) | 0.002 |
| Mood disorders, n (%) | 5 (4.1) | 0 | 5 (18.5) | <0.001 |
| Anxiety disorders, n (%) | 4 (3.3) | 3 (3.2) | 1 (3.7) | 1 |
| Photophobia, n (%) | 6 (4.9) | 3 (3.2) | 3 (11.1) | 0.122 |
| Phonophobia, n (%) | 1 (0.8) | 0 | 1 (3.7) | N.A. |
| Meningeal signs, n (%) | 5 (4.1) | 1 (1.1) | 4 (14.8) | 0.008 |
| Bulging fontanelle, n (%) | 2 (1.6) | 1 (1.1) | 1 (3.7) | 0.395 |
| Dizziness, n (%) | 5 (4.1) | 3 (3.2) | 2 (7.4) | 0.306 |
| Dysgeusia/ageusia, n (%) | 4 (3.3) | 1 (1.1) | 3 (11.1) | 0.034 |
| Hyper/hypotonia, n (%) | 4 (3.3) | 4 (4.2) | 0 | 0.575 |
| Balance deficit, n (%) | 3 (2.5) | 3 (3.2) | 0 | 1 |
| Gait alterations, n (%) | 3 (2.5) | 3 (3.2) | 0 | 1 |
| Motor deficit, n (%) | 2 (1.6) | 2 (2.1) | 0 | 1 |
| Retrograde amnesia, n (%) | 2 (1.6) | 2 (2.1) | 0 | 1 |
| Speech disturbances, n (%) | 2 (1.6) | 0 | 2 (7.4) | 0.048 |
| Visual hallucinations, n (%) | 1 (0.8) | 1 (1.1) | 0 | N.A. |
| Visual impairment, n (%) | 3 (2.5) | 2 (2.1) | 1 (3.7) | 0.531 |
| Nystagmus, n (%) | 3 (2.5) | 3 (3.2) | 0 | 1 |
| Strabismus, n (%) | 1 (0.8) | 1 (1.1) | 0 | N.A. |
| Double vision, n (%) | 1 (0.8) | 1 (1.1) | 0 | N.A. |
| Others: Sleeping disorders, n (%) | 2 (1.6) | 1 (1.1) | 1 (3.7) | 0.395 |
| Factitious disorder, n (%) | 1 (0.8) | 1 (1.1) | 0 | N.A. |
| Neuropathic pain, n (%) | 1 (0.8) | 1 (1.1) | 0 | N.A. |
| Osteotendineous reflexes deficit, n (%) | 1 (0.8) | 1 (1.1) | 0 | N.A. |
| Tests | Total (n = 122) | COVID-19 (n = 95) | MIS-C (n = 27) | p |
|---|---|---|---|---|
| EEG, n (%) | 4 (3.3) | 2 (2.1) Diffuse symmetric slow wave activity (1) centro-temporal spikes (BRE/CECTS) (1) | 2 (7.4) Diffuse symmetric slow wave activity (2) | 0.212 |
| ENG/EMG, n (%) | 1 (0.8) | 1 (1.1) NCV slowing, absent F waves | 0 | N.A |
| Flash VEP, n (%) | 1 (0.8) | 1 (1.1) Left optic NCV impairment | 0 | N.A |
| TCS/TCD, n (%) | 0 | 0 | 0 | N.A. |
| Brain CT, n (%) | 2 (1.6) | 1 (1.1) Parenchimal hypodensity | 1 (3.7) Brain oedema and herniation | 0.395 |
| Brain/spinal cord MRI, n (%) | 5 (4.1) | 3 (3.2) Brain ischemic lesions (2), cerebral artery occlusion (1), contrast enhancement cauda equina (1) | 2 (7.4) Cranial nerve contrast enhancement (1) cortical/subcortical atrophy (2), periventricular/ peritrigonal hyperintense signal (1) | 0.306 |
| DSA, n (%) | 1 (0.8) | 1 (1.1) Partial left PICA occlusion | 0 | N.A. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbati, G.; Attaianese, F.; Rosati, A.; Indolfi, G.; Trapani, S. Neurological Involvement in Children with COVID-19 and MIS-C: A Retrospective Study Conducted for More than Two Years in a Pediatric Hospital. Children 2022, 9, 1809. https://doi.org/10.3390/children9121809
Abbati G, Attaianese F, Rosati A, Indolfi G, Trapani S. Neurological Involvement in Children with COVID-19 and MIS-C: A Retrospective Study Conducted for More than Two Years in a Pediatric Hospital. Children. 2022; 9(12):1809. https://doi.org/10.3390/children9121809
Chicago/Turabian StyleAbbati, Giulia, Federica Attaianese, Anna Rosati, Giuseppe Indolfi, and Sandra Trapani. 2022. "Neurological Involvement in Children with COVID-19 and MIS-C: A Retrospective Study Conducted for More than Two Years in a Pediatric Hospital" Children 9, no. 12: 1809. https://doi.org/10.3390/children9121809
APA StyleAbbati, G., Attaianese, F., Rosati, A., Indolfi, G., & Trapani, S. (2022). Neurological Involvement in Children with COVID-19 and MIS-C: A Retrospective Study Conducted for More than Two Years in a Pediatric Hospital. Children, 9(12), 1809. https://doi.org/10.3390/children9121809






