Diagnostic Validity and Reliability of Low-Dose Prospective ECG-Triggering Cardiac CT in Preoperative Assessment of Complex Congenital Heart Diseases (CHDs)
Abstract
:1. Introduction
2. Patients and Methods
2.1. Ethical Statement
2.2. Study Population
2.3. Cardiac CT Acquisition
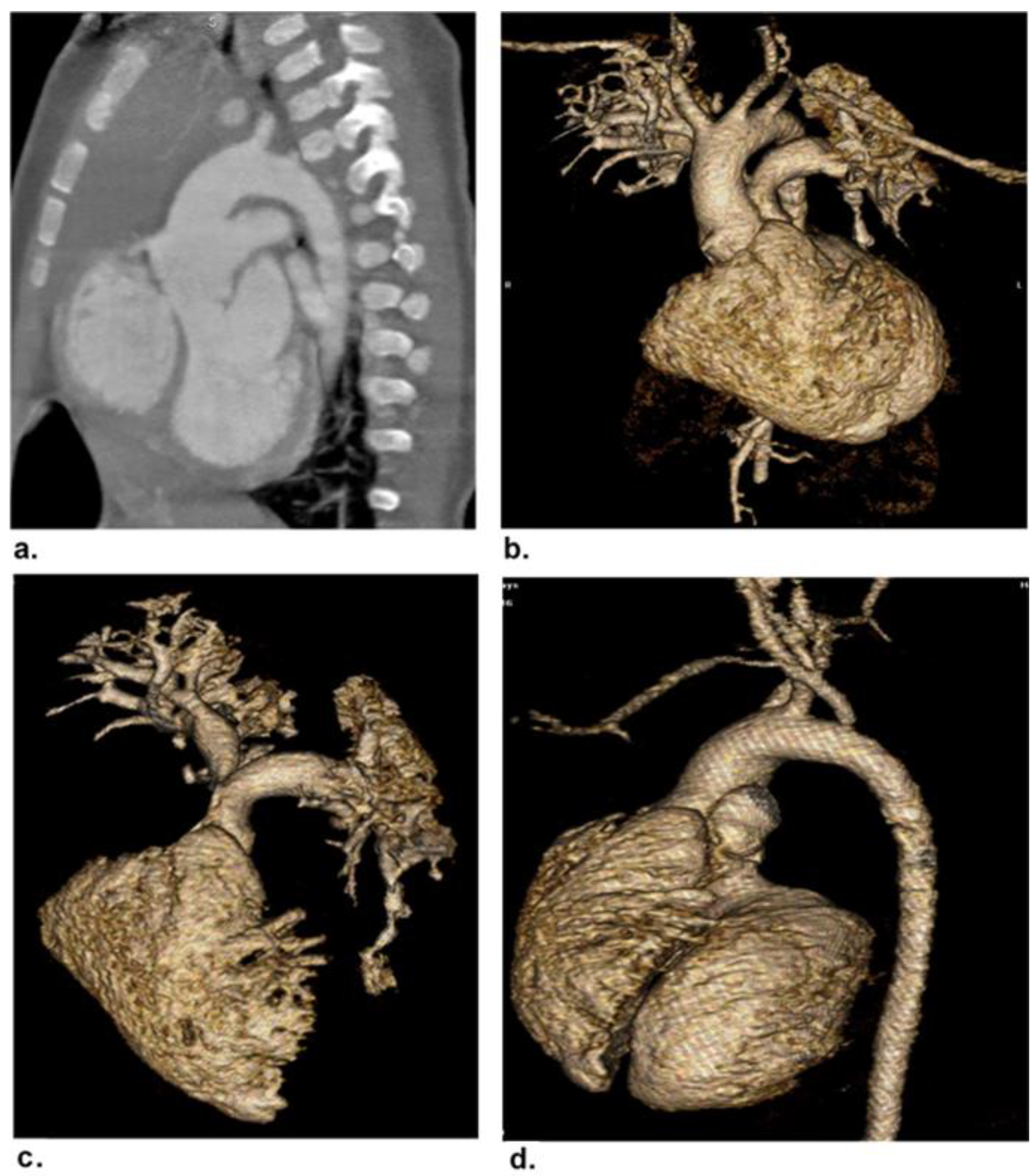
2.4. Image Post-Processing and Interpretation
- a.
- Evaluation of imaging quality
- b.
- Coronary artery evaluation
2.5. Radiation Dose Estimation
2.6. Reference Standard
2.7. Statistical Analysis
3. Result
3.1. Patients and CHDs
3.2. Coronary Artery Evaluation
3.3. Diagnostic Validity of Cardiac CT
3.4. Reliability of Cardiac CT
3.5. Image Quality Evaluation
3.6. Radiation Dose Estimation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miranovic, V. The incidence of congenital heart disease: Previous findings and perspectives. Srp. Arh. Celok. Lek. 2014, 142, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Buratto, E.; Ye, X.-T.; Konstantinov, I.E. Simple congenital heart disease: A complex challenge for public health. J. Thorac. Dis. 2016, 8, 2994–2996. [Google Scholar] [CrossRef] [Green Version]
- Roest, A.A.W.; De Roos, A. Imaging of patients with congenital heart disease. Nat. Rev. Cardiol. 2012, 9, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-P.; Liang, C.-H.; Zhao, Z.-J.; Liu, H.; Li, J.-L.; Zhang, J.-E.; Cui, Y.-H.; Yang, L.; Liu, Q.-S.; Ivanc, T.B.; et al. Evaluation of image quality and radiation dose at prospective ECG-triggered axial 256-slice multi-detector CT in infants with congenital heart disease. Pediatr. Radiol. 2011, 41, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Goo, H.W.; Seo, D.-M.; Yun, T.-J.; Park, J.-J.; Park, I.-S.; Ko, J.K.; Kim, Y.H. Coronary artery anomalies and clinically important anatomy in patients with congenital heart disease: Multislice CT findings. Pediatr. Radiol. 2009, 39, 265–273. [Google Scholar] [CrossRef]
- Raimondi, F.; Warin-Fresse, K. Computed tomography imaging in children with congenital heart disease: Indications and radiation dose optimization. Arch. Cardiovasc. Dis. 2016, 109, 150–157. [Google Scholar] [CrossRef] [Green Version]
- Listijono, D.R.; Rubens, M.B.; Rigby, M.L. Complementary Use of Imaging modalities in Diagnosis of Complex Con-genital Heart Disease. ASEAN Heart J. 2014, 22, 1. [Google Scholar] [CrossRef] [Green Version]
- Bu, G.; Miao, Y.; Bin, J.; Deng, S.; Liu, T.; Jiang, H.; Chen, W. Comparison of 128-Slice Low-Dose Prospective ECG-Gated CT Scanning and Trans-Thoracic Echocardiography for the Diagnosis of Complex Congenital Heart Disease. PLoS ONE 2016, 11, e0165617. [Google Scholar] [CrossRef] [Green Version]
- Charakida, M.; Pushparajah, K.; Simpson, J. 3D echocardiography in congenital heart disease: A valuabletool for the surgeon. Future Cardiol. 2014, 10, 497–509. [Google Scholar] [CrossRef]
- Tricarico, F.; Hlavacek, A.; Schoepf, U.J.; Ebersberger, U.; Nance, J.W.; Vliegenthart, R.; Cho, Y.J.; Spears, J.R.; Secchi, F.; Savino, G.; et al. Cardiovascular CT angiography in neonates and children: Image quality and potential for radiation dose reduction with iterative image reconstruction techniques. Eur. Radiol. 2013, 23, 1306–1315. [Google Scholar] [CrossRef]
- Driessen, M.M.P.; Breur, J.M.P.J.; Budde, R.P.J.; Van Oorschot, J.W.M.; Van Kimmenade, R.R.J.; Sieswerda, G.T.; Meijboom, F.J.; Leiner, T. Advances in cardiac magnetic resonance imaging of congenital heart disease. Pediatr. Radiol. 2015, 45, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Pache, G.; Grohmann, J.; Bulla, S.; Arnold, R.; Stiller, B.; Schlensak, C.; Langer, M.; Blanke, P. Prospective electrocardiography-triggered CT angiography of the great thoracic vessels in infants and toddlers with congenital heart disease: Feasibility and image quality. Eur. J. Radiol. 2011, 80, e440–e445. [Google Scholar] [CrossRef] [PubMed]
- Entrikin, D.W.; Leipsic, J.A.; Carr, J.J. Optimization of radiation dose reduction in cardiac computed tomographic angi-ography. Cardiol. Rev. 2011, 19, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.-C.; Lee, T.; Chen, M.-C.; Fu, Y.-C.; Jan, S.-L.; Wang, C.-C.; Chang, Y. Visualization of neonatal coronary arteries on multidetector row CT: ECG-gated versus non-ECG-gated technique. Pediatr. Radiol. 2007, 37, 818–825. [Google Scholar] [CrossRef]
- Nie, P.; Wang, X.; Cheng, Z.; Ji, X.; Duan, Y.; Chen, J. Accuracy, image quality and radiation dose comparison of high-pitch spiral and sequential acquisition on 128-slice dual-source CT angiography in children with congenital heart disease. Eur. Radiol. 2012, 22, 2057–2066. [Google Scholar] [CrossRef]
- Ghoshhajra, B.B.; Lee, A.M.; Engel, L.C.; Celeng, C.; Kalra, M.K.; Brady, T.J.; Hoffmann, U.; Westra, S.J.; Abbara, S. Radiation Dose Reduction in Pediatric Cardiac Computed Tomography: Ex-perience from a Tertiary Medical Center. Pediatr. Cardiol. 2014, 35, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Sigal-Cinqualbre, A.; Lambert, V.; Ronhean, A.; Paul, J.F. Role of MSCT and MRI in the diagnosis of congenital heart disease. Arch. Pediatr. Organe Off. Soc. Fr. Pediatr. 2011, 18, 617–627. [Google Scholar] [CrossRef]
- Goske, M.J.; Applegate, K.E.; Bulas, D.; Butler, P.F.; Callahan, M.J.; Coley, B.D.; Don, S.; Frush, D.P.; Hernanz-Schulman, M.; Kaste, S.C.; et al. Image Gently: Progress and challenges in CT education and advocacy. Pediatr. Radiol. 2011, 41, 461–466. [Google Scholar] [CrossRef]
- Stinn, B.; Stolzmann, P.; Fornaro, J.; Hibbeln, D.; Alkadhi, H.; Wildermuth, S.; Leschka, S. Technical principles of computed tomography in patients with congenital heart disease. Insights Imaging 2011, 2, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Engel, L.C.; Ferencik, M.; Liew, G.Y.; Karolyi, M.; Sidhu, M.S.; Lee, A.M.; Wai, B.; Blankstein, R.; Abbara, S.; Hoffmann, U.; et al. Ultra-low dose cardiac CT angiography at 80 kV using second generation dual- source CT: Assessment of radiation dose and image quality. J. Med. Diagn Methods 2012, 1, 104. [Google Scholar] [CrossRef]
- Strauss, K.J.; Goske, M.J.; Kaste, S.C.; Bulas, D.; Frush, D.P.; Butler, P.; Morrison, G.; Callahan, M.J.; Applegate, K.E. Image Gently: Ten Steps You Can Take to Optimize Image Quality and Lower CT Dose for Pediatric Patients. Am. J. Roentgenol. 2010, 194, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Bruesewitz, M.R.; Thomas, K.B.; Fletcher, J.G.; Kofler, J.M.; McCollough, C.H. Optimal Tube Potential for Radiation Dose Reduction in Pediatric CT: Principles, Clinical Implementations, and Pitfalls. RadioGraphics 2011, 31, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.J.; Kim, Y.J.; Hong, S.R.; Hong, Y.J.; Lee, H.-J.; Hur, J.; Choi, B.W. Combined Use of Automatic Tube Potential Selection with Tube Current Modulation and Iterative Reconstruction Technique in Coronary CT Angiography. Radiology 2013, 269, 722–729. [Google Scholar] [CrossRef]
- Krazinski, A.W.; Meinel, F.; Schoepf, U.J.; Silverman, J.R.; Canstein, C.; De Cecco, C.N.; Geyer, L.L. Reduced radiation dose and improved image quality at cardiovascular CT angiography by automated attenuation-based tube voltage selection: Intra-individual comparison. Eur. Radiol. 2014, 24, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Gao, W.; Zhong, Y.-M.; Sun, A.-M.; Wang, Q.; Hu, L.-W.; Qiu, H.-S.; Li, J.-Y. Prospective ECG-triggering cardiac CT for infants with complex congenital heart disease using low-dose contrast medium, low tube voltage, and adaptive statistical iterative reconstruction. Clin. Radiol. 2017, 72, 502–507. [Google Scholar] [CrossRef]
- Herzog, C.; Mulvihill, D.M.; Nguyen, S.A.; Savino, G.; Schmidt, B.; Costello, P.; Vogl, T.J.; Schoepf, U.J. Pediatric Cardiovascular CT Angiography: Radiation Dose Reduction Using Automatic Anatomic Tube Current Modulation. Am. J. Roentgenol. 2008, 190, 1232–1240. [Google Scholar] [CrossRef] [Green Version]
- Boone, J.M.; Geraghty, E.M.; Seibert, J.A.; WoottonGorges, S.L. Dose reduction in pediatric CT: A rational approach. Radiology 2003, 228, 352–360. [Google Scholar] [CrossRef]
- Podberesky, D.J.; Angel, E.; Yoshizumi, T.T.; Toncheva, G.; Salisbury, S.R.; Alsip, C.; Barelli, A.; Egelhoff, J.C.; Anderson-Evans, C.; Nguyen, G.B.; et al. Radiation Dose Estimation for Prospective and Retrospective ECG-Gated Cardiac CT Angiography in Infants and Small Children Using a 320-MDCT Volume Scanner. Am. J. Roentgenol. 2012, 199, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhong, Y.M.; Sun, A.M.; Wang, Q.; Ouyang, R.Z.; Hu, L.W.; Qiu, H.S.; Wang, S.Y.; Li, J.Y. Diagnostic accuracy of sub-mSv prospective ECG-triggering cardiac CT in young infant with complex congenital heart disease. Int. J. Cardiovasc. Imaging 2016, 32, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.-F.; Rohnean, A.; Elfassy, E.; Sigal-Cinqualbre, A. Radiation dose for thoracic and coronary step-and-shoot CT using a 128-slice dual-source machine in infants and small children with congenital heart disease. Pediatr. Radiol. 2010, 41, 244–249. [Google Scholar] [CrossRef]
- Sorantin, E.; Riccabona, M.; Stucklschweiger, G.; Guss, H.; Fotter, R. Experience with volumetric (320 rows) pediatric CT. Eur. J. Radiol. 2012, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Huang, M.; Zheng, J.; Li, J.; Liu, H.; Liang, C. Assessments of Coronary Artery Visibility and Radiation Dose in Infants with Congenital Heart Disease on Cardiac 128-slice CT and on Cardiac 64-slice CT. Pediatr. Cardiol. 2016, 37, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Ghoshhajra, B.B.; Engel, L.C.; Karolyi, M.; Manavjot Singh, S.; Bryan, W.; Mitya, B.; Uthamalingam, S.; Udo, H.; Thomas, J.B.; Manudeep, K.; et al. Cardiac computed tomography angiography with automatic tube potential selection: Effects on radiation dose and image quality. J. Thorac. Imagings 2012, 28, 40–48. [Google Scholar] [CrossRef]
- Leipsic, J.; Labounty, T.M.; Heilborn, B.; Min, J.K.; Mancini, G.B.J.; Lin, F.Y.; Taylor, C.; Dunning, A.; Earls, J.P. Estimated radiation dose reduction using adaptive statistical iterative recon-struction in coronary CT angiography: The ERASIR study. AJR Am J. Roentgenol. 2010, 195, 655–660. [Google Scholar] [CrossRef]
- Jin, K.N.; Park, E.-A.; Shin, C.-I.; Lee, W.; Chung, J.W.; Park, J.H. Retrospective versus prospective ECG-gated dual-source CT in pediatric patients with congenital heart diseases: Comparison of image quality and radiation dose. Int. J. Cardiovasc. Imaging 2010, 26, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Hossain, M.; Khaleque, M.; Das, M.; Khan, M.; Bari, M.; Bhuiyan, M. Prevalence of Congenital Heart Disease in Neonate in a Tertiary Level Hospital. Nepal J. Med. Sci. 2013, 2, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Mita, S.A.; Salim; Haque, A.; Shahriar, A.; Zakia, N.U.; Salam, A.A.; Rahman, A.R.; Jafar, A.H. Comorbidities with Congenital Heart Disease among Hospitalized Children in a Specialized Cardiac Hospital in Bangladesh. Cardiovasc. J. 2017, 9, 83–89. [Google Scholar] [CrossRef]


| Body Weight (kg) | Tube Voltage (kV) | Tube Current (mAs) |
|---|---|---|
| 0–3 | 80 | 60 |
| 3.1–6 | 80 | 80 |
| 6.1–10 | 80 | 100 |
| >10 | 80 | 120 |
| Variable | Value |
|---|---|
| Age, months | 27.3 ± 8.1 (3 days–16 years) |
| Age groups, number (%) Neonates (1–30 days) Infants (31 days–2 years) Preschool children (2–6 years) School children (6–12 years) Adolescents (>12 years) | 12 (28.6) 19 (45.2) 5 (11.9) 4 (9.5) 2 (4.8) |
| Sex, number (%) | |
| Male | 22 (52.4) |
| Female | 20 (47.6) |
| Variable | Value |
|---|---|
| Clinical Weight (gm), mean ± SD (range) Heart rate (beat/min), mean ± SD (range) Cyanosis (%) Chest troubles (%) Dyspnea (%) Delayed milestone (%) Edema (%) Ascites (%) Laboratory Hemoglobin (g/dL), mean ± SD Hematocrit, mean ± SD Cholesterol, (mg/dL), mean ± SD Glucose, (mg/dL), mean ± SD C-reactive protein, (mg/dL), mean ± SD Associated comorbidities and extracardiac malformations Hypertension (%) Heart disease/ischemic disease (%) Obesity (%) Diabetes (%) Dyslipidemia (%) Pneumonia (%) Down’s syndrome (%) Cleft lip or Cleft palate (%) Hirschprung disease (%) Esophageal atresia (%) Mental retardation (%) Scoliosis (%) Renal dysplasia (%) | 10,026.2 ± 1085.1 (1400–45,000) 123.9 ± 20.1 (75–160) 17 (40.5) 24 (57.1) 9 (21.4) 15 (35.7) 7 (16.7) 4 (9.5%) 13.1± 1.5 38.9 ± 3.1 145.4 ± 51.8 81.2 ± 10.2 0.2 ± 0.1 4 (9.5) 4 (9.5) 3 (7.1) 3 (7.1) 2 (4.8) 3 (7.1) 5 (11.9) 2 (4.8) 1 (2.4) 2 (4.8) 2 (4.8) 1 (2.4) 1 (2.4) |
| CHDs | Number of Patients (%) |
|---|---|
| Intracardiac/Cardiac Malposition Ventricular septal defect Atrial septal defect Dextrocardia | 8 (11.8) 6 (8.8) 2 (2.9) |
| Conotruncal Tetralogy of Fallot Truncus arteriosus | 7 (10.3) 3 (4.4) |
| Abnormal Connection Transposition of great arteries Double outlet right ventricle | 2 (2.9) 2 (2.9) |
| Extracardiac Total anomalous pulmonary venous return Aortic coarctation Patent ductus arteriosus Double aortic arch Supravalvular aortic stenosis Pulmonary artery stenosis Pulmonary atresia Coronary artery anomalies | 4 (5.9) 6 (8.8) 10 (14.7) 1 (1.5) 3 (4.4) 7 (10.3) 3 (4.4) 4 (5.9) |
| Total | 68 |
| Diagnostic Rate | Quality Score Mean ± SD (Range) | |
|---|---|---|
| LMCA | 42 | 4.47 ± 0.5 (4–5) |
| LAD Proximal Mid Distal | 42 40 36 | 4.42 ± 0.5 (4–5) 3.6 ± 0.9 (1–5) 2.7± 0.8 (1–4) |
| LCX Proximal Distal | 42 30 | 4.19 ± 0.55 (3–5) 2.3 ± 0.9 (1–4) |
| RCA Proximal Mid Distal | 42 35 31 | 3.78 ± 0.6 (3–5) 2.8 ± 0.7 (1–4) 2.8 ± 0.8 (2–4) |
| Overall | 42 | 3.63 ± 0. 65 (2.2–4.8) |
| Reader 1 | Reader 2 | |
|---|---|---|
| Intracardiac/cardiac malposition anomaly | ||
| Accuracy % | 92.9 [80.5–98.5] | 92.9 [80.5–98.5] |
| Sensitivity % | 93.8 [69.7–99.8] | 87.5 [61.6–98.5] |
| Specificity % | 92.3 [74.9–99.1] | 96.2 [80.4–99.9] |
| PPV % | 88.2 [66.3–96.6] | 93.3 [67–98.9] |
| NPV % | 96 [78.2–99.3] | 92.6 [77.3–97.9] |
| AUC | 0.93 | 0.91 |
| Conotruncal anomaly | ||
| Accuracy % | 100 [91.6–100] | 95.2 [83.8–99.4] |
| Sensitivity % | 100 [69.2–100.0] | 90 [55.5–99.7] |
| Specificity % | 100 [89.1–100] | 96.9 [83.8–99.9] |
| PPV % | 100 | 90 [56.4–98.4] |
| NPV % | 100 | 96.9 [82.3–99.5] |
| AUC | 1 | 0.93 |
| Abnormal connection anomaly | ||
| Accuracy % | 100 [91.6–100] | 95.2 [83.8–99.4] |
| Sensitivity % | 100 [39.8–100] | 75 [19.4–99.4] |
| Specificity % | 100 [90.7–100] | 97.4 [86.2–99.49 |
| PPV % | 100 | 75 [28.6–95.7] |
| NPV % | 100 | 97.3 [87.1–99.5] |
| AUC | 1 | 0.86 |
| Extra-cardiac anomaly | ||
| Accuracy % | 95.2 [83.8–99.4] | 97.6 [87.4–99.9] |
| Sensitivity % | 94.7 [82.2–99.4] | 97.4 [86.2–99.9] |
| Specificity % | 100 [39.8–100] | 100 [39.8–100] |
| PPV % | 100 | 100 |
| NPV % | 66.7 [34.2–88.5] | 80 [36.6–96.5] |
| AUC | 0.97 | 0.99 |
| Overall | ||
| Accuracy % | 97 [93.2–99] | 95.2 [90.8–97.9] |
| Sensitivity % | 95.6 [87.6–99.1] | 92.6 [83.7–97.6] |
| Specificity % | 98 [92.9–99.8] | 97 [91.5–99.4] |
| PPV % | 97 [89.2–99.2] | 95.5 [87.3–98.5] |
| NPV % | 97 [91.5–98.9] | 95.1 [89.3–97.8] |
| AUC | 0.97 | 0.95 |
| Variable | Value |
|---|---|
| Intra-cardiac anomaly | 0.89 (0.76–1.00) |
| Conotruncal anomaly | 0.94 (.81–1.00) |
| Abnormal connection anomaly | 0.88 (0.64–1.00) |
| Extra-cardiac anomaly Image quality | 0.77 (0.48–1.00) 0.78 (0.51–1.00) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almalki, Y.E.; Basha, M.A.A.; Alduraibi, S.K.; Alshamrani, K.; Huneif, M.A.; Alduraibi, A.K.; Almedhesh, S.A.; Alshamrani, H.A.; Elbanna, K.A.A.; Algazzar, Y.H.; et al. Diagnostic Validity and Reliability of Low-Dose Prospective ECG-Triggering Cardiac CT in Preoperative Assessment of Complex Congenital Heart Diseases (CHDs). Children 2022, 9, 1903. https://doi.org/10.3390/children9121903
Almalki YE, Basha MAA, Alduraibi SK, Alshamrani K, Huneif MA, Alduraibi AK, Almedhesh SA, Alshamrani HA, Elbanna KAA, Algazzar YH, et al. Diagnostic Validity and Reliability of Low-Dose Prospective ECG-Triggering Cardiac CT in Preoperative Assessment of Complex Congenital Heart Diseases (CHDs). Children. 2022; 9(12):1903. https://doi.org/10.3390/children9121903
Chicago/Turabian StyleAlmalki, Yassir Edrees, Mohammad Abd Alkhalik Basha, Sharifa Khalid Alduraibi, Khalaf Alshamrani, Mohammed Ayed Huneif, Alaa Khalid Alduraibi, Sultan A. Almedhesh, Hassan A. Alshamrani, Khaled Ahmed Ahmed Elbanna, Youssef H. Algazzar, and et al. 2022. "Diagnostic Validity and Reliability of Low-Dose Prospective ECG-Triggering Cardiac CT in Preoperative Assessment of Complex Congenital Heart Diseases (CHDs)" Children 9, no. 12: 1903. https://doi.org/10.3390/children9121903






