Diagnosing Single and Multiple Drug Hypersensitivity in Children: A Tertiary Care Center Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Analysis of Confirmed DHRs
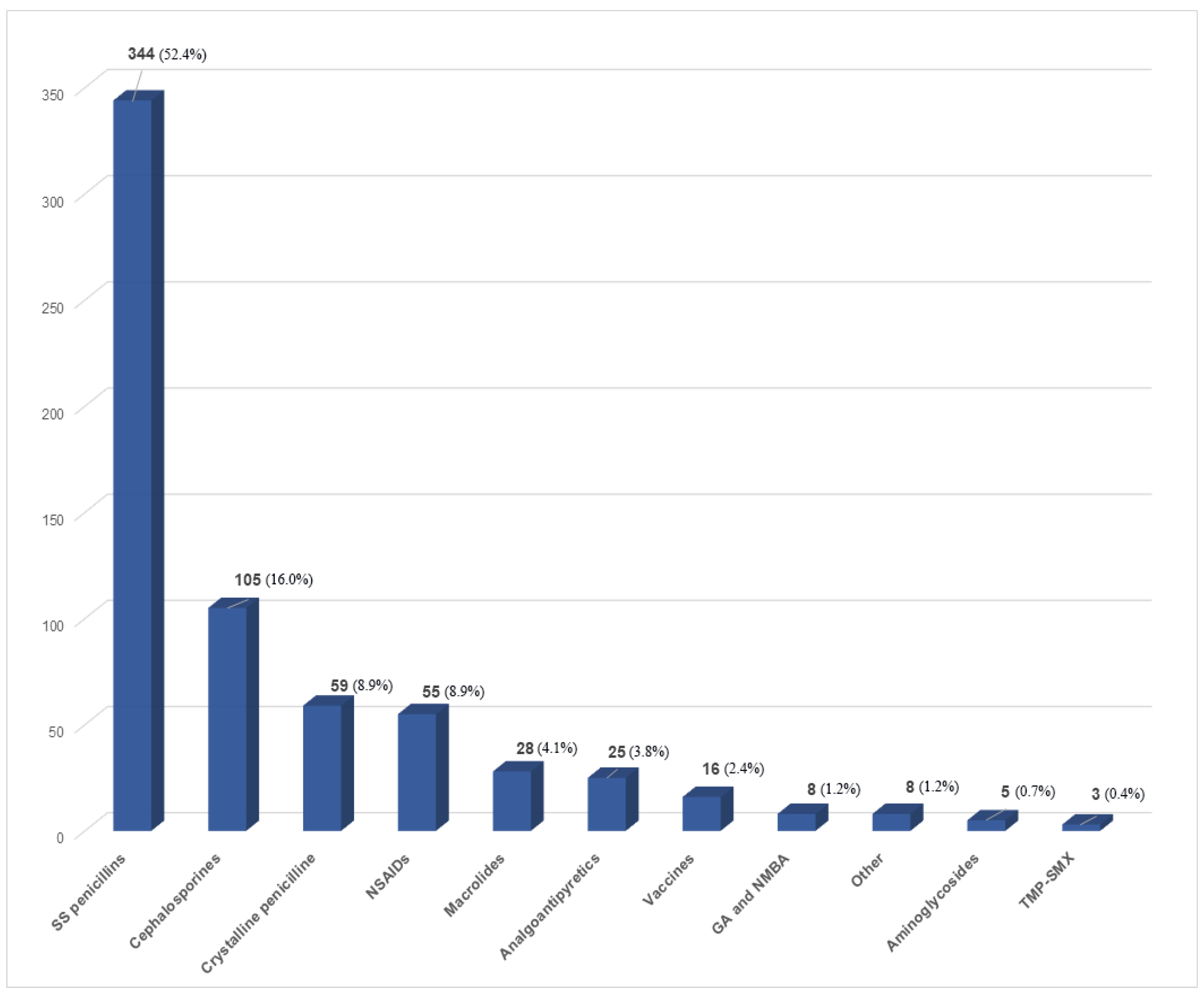
3.1.1. DHRs to Antibiotics
3.1.2. DHRs to Analgoantipyretics (AA)
3.1.3. DHRs to General Anesthetics (GA) and Neuromuscular Blocking Agents (NMBA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edwards, I.R.; Aronson, J.K. Adverse drug reactions: Definitions, diagnosis, and management. Lancet 2000, 356, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; In Suh, D. Drug allergy in children: What should we know? Korean J. Pediatrics 2020, 63, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Atanasković-Marković, M.; Gaeta, F.; Gavrović-Jankulović, M.; Čirković Veličković, T.; Valluzzi, R.L.; Romano, A. Diagnosing multiple drug hypersensitivity in children. Pediatric Allergy Immunol. 2012, 23, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.R.; Brockow, K.; Kuyucu, S.; Saretta, F.; Mori, F.; Blancalopez, N.; Ott, H.; Atanaskovicmarkovic, M.; Kidon, M.I.; Caubet, J.-C.R.J.-P.; et al. Drug hypersensitivity in children: Report from the pediatric task force of the EAACI Drug Allergy Interest Group. Allergy: European J. Allergy Clin. Immunol. 2016, 71, 149–161. [Google Scholar] [CrossRef]
- Demoly, P.; Adkinson, N.F.; Brockow, K.; Castells, M.; Chiriac, A.M.; Greenberger, P.A.; Khan, D.A.; Lang, D.M.; Park, H.-S.; Pichler, W.J.; et al. International Consensus on drug allergy. Allergy Eur. J. Allergy Clin. Immunol. 2014, 69, 420–437. [Google Scholar] [CrossRef]
- Brockow, K.; Garvey, L.H.; Aberer, W.; Atanaskovic-Markovic, M.; Barbaud, A.; Bilo, M.B.; Bircher, A.; Blanca, M.; Bonadonna, B.; Campi, P.; et al. Skin test concentrations for systemically administered drugs - An ENDA/EAACI Drug Allergy Interest Group position paper. Allergy: Eur. J. Allergy Clin. Immunol. 2013, 68, 702–712. [Google Scholar] [CrossRef]
- Brockow, K.; Romano, A.; Blanca, M.; Ring, J.; Pichler, W.; Demoly, P. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy: Eur. J. Allergy Clin. Immunol. 2002, 57, 45–51. [Google Scholar] [CrossRef]
- Romano, A.; Blanca, M.; Torres, M.J.; Bircher, A.; Aberer, W.; Brockow, K.; Pichler, W.J.; Demoly, P. Diagnosis of nonimmediate reactions to β-lactam antibiotics. Allergy: Eur. J. Allergy Clin. Immunol. 2004, 59, 1153–1160. [Google Scholar] [CrossRef]
- Caubet, J.C.; Eigenmann, P.A. Managing possible antibiotic allergy in children. Curr. Opin. Infect. Dis. 2012, 25, 279–285. [Google Scholar] [CrossRef]
- Piccorossi, A.; Liccioli, G.; Barni, S.; Sarti, L.; Giovannini, M.; Verrotti, A.; Novembre, E.; Mori, F. Epidemiology and drug allergy results in children investigated in allergy unit of a tertiary-care paediatric hospital setting. Ital. J. Pediatrics 2020, 46, 1–13. [Google Scholar] [CrossRef]
- Atanaskovic-Markovic, M.; Caubet, J.C. Management of drug hypersensitivity in the pediatric population. Expert Rev. Clin. Pharmacol. 2016, 9, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Impicciatore, P.; Choonara, I.; Clarkson, A.; Provasi, D.; Pandolfini, C.; Bonati, M. Incidence of adverse drug reactions in paediatric in/out-patients: A systematic review and meta-analysis of prospective studies. Br. J. Clin. Pharmacol. 2001, 52, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Buonomo, A.; Altomonte, G.; De Pasquale, T.; Lombardo, C.; Pecora, V.; Sabato, V.; Colagiovanni, A.; Rizzi, A.; Aruanno, A.; Pascolini, L.; et al. Allergic and non-allergic drug hypersensitivity reactions in children. Int. J. Immunopathol. Pharmacol. 2010, 23, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Vezir, E.; Erkocoglu, M.; Civelek, E.; Kaya, A.; Azkur, D.; Akan, A.; Ozcan, C.; Toyran, M.; Ginis, T.; Misirlioglu, E.D.; et al. The evaluation of drug provocation tests in pediatric allergy clinic: A single center experience. Allergy Asthma Proc. 2014, 35, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Statistical Yearbook of the Republic of Serbia. Belgrade, Serbia: Statistical Office of the Republic of Serbia. 2018. Available online: https://www.stat.gov.rs/en-us/publikacije/publication/?p=11525 (accessed on 1 July 2022).
- Kamada, M.M.; Twarog, F.; Leung, D.Y.M. Multiple antibiotic sensitivity in a pediatric population. Allergy Proc. 1991, 12, 347–350. [Google Scholar] [CrossRef]
- Guvenir, H.; Misirlioglu, E.D.; Toyran, M.; Civelek, E.; Buyuktiryaki, B.; Ginis, T.; Kocabas, C.N. immunologically-mediated drug hypersensitivity in children with a history of multiple drug intolerances. Ann. Allergy Asthma Immunol. 2019, 122, 73–78. [Google Scholar] [CrossRef]
- Goldberg, R.M.; Mabee, J.; Chan, L.; Wong, S. Drug-Drug and Drug-Disease Interactions in the ED: Analysis of a High-Risk Population. Am. J. Emerg. Med. 1996, 14, 447–450. [Google Scholar] [CrossRef]
- Goh, S.H.; Chong, K.W.; Chiang, W.C.; Goh, A.; Loh, W. Outcome of drug provocation testing in children with suspected beta-lactam hypersensitivity. Asia Pac. Allergy 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Rieder, M. Adverse drug reactions in children: Pediatric pharmacy and drug safety. J. Pediatric Pharmacol. Ther. 2019, 24, 4–9. [Google Scholar] [CrossRef]
- Norton, A.E.; Konvinse, K.; Phillips, E.J.; Broyles, A.D. Antibiotic allergy in pediatrics. Pediatrics 2018, 141, e20172497. [Google Scholar] [CrossRef]
- MacLaughlin, E.J.; Saseen, J.J.; Malone, D.C. Costs of beta-lactam allergies: Selection and costs of antibiotics for patients with a reported beta-lactam allergy. Arch. Fam. Med. 2000, 9, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.; Romeira, A.M.; Abreu, C.; Carreiro-Martins, P.; Gomes, E.; Leiria-Pinto, P. Non-steroidal anti-inflammatory drug hypersensitivity in children. Allergologia et Immunopathologia 2017, 45, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Uribe, V.; Navarrete Rodriguez, E.M. Allergic Reactions in the Perioperative Period in Children. J. Allergy Ther. 2016, 7, 238. [Google Scholar] [CrossRef]
- Cheung, A.; Perrett, K.P. Immunisation and allergy in children and adults. Aust. J. Gen. Pract. 2020, 49, 637–647. [Google Scholar] [CrossRef]
- Erkoçoğlu, M.; Kaya, A.; Civelek, E.; Özcan, C.; Çakır, B.; Akan, A.; Toyran, M.; Ginis, T.; Kocabas, C.N. Prevalence of confirmed immediate type drug hypersensitivity reactions among school children. Pediatric Allergy Immunol. 2013, 24, 160–167. [Google Scholar] [CrossRef]

| Drug Class | Suspected Adverse Reactions | |||||||
|---|---|---|---|---|---|---|---|---|
| Cutaneous (C) | Total C Adrs within Group | Extracutaneous (EC) | Total EC ADRs within Group | |||||
| Urticaria (%) * | Exanthema (%) * | Angioedema (%) * | Dyspnea (%) * | Syncope (%) * | Other **** (%) * | |||
| Aminoglycosides | 2 (40) | 2 (40) | 1 (20) | 5 | 0 | 0 | 0 | |
| Analgoantipyretics | 14 (56) | 5 (20) | 6 (24) | 25 | 0 | 0 | 0 | |
| Cephalosporines | 52 (50) | 43 (41.3) | 9 (8.7) | 104 | 2 (50) | 0 | 2 (50) | 4 |
| Crystalline penicillins | 21 (38.9) | 29 (53.7) | 4 (7.4) | 54 | 0 | 3 (60) | 2 (40) | 5 |
| Macrolides | 13 (46.4) | 11 (39.3) | 4 (14.3) | 28 | 0 | 0 | 0 | |
| NSAIDs | 24 (44.4) | 11 (20.4) | 19 (35.2) | 54 | 1 *** (50) | 0 | 1 (50) | 2 |
| GA and NMBA | 1 (20) | 2 (40) | 2 (40) | 5 | 3 (100) | 0 | 0 | 3 |
| Other | 3 (37.5) | 3 (37.5) | 2 (25) | 8 | 0 | 0 | 0 | |
| Semi-synthetic penicillins | 165 (48) | 151 (43.9) | 28 (8.1) | 344 | 2 (66.7) | 1 (33.3) | 0 | 3 |
| TMP/SMX | 2 (66.7) | 1 (33.3) | 0 | 3 | 0 | 0 | 0 | |
| Vaccines | 4 (44.4) | 2 (22.2) | 3 (33.3) | 9 | 0 | 0 | 8 (100) | 8 |
| Total ADRs ** | 301 (45.3) | 260 (39.2) | 78 (11.7) | 639 (96.2) | 8 (1.2) | 4 (0.6) | 13 (1.96) | 25 (3.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milosevic, K.; Malinic, M.; Plavec, D.; Lekovic, Z.; Lekovic, A.; Cobeljic, M.; Rsovac, S. Diagnosing Single and Multiple Drug Hypersensitivity in Children: A Tertiary Care Center Retrospective Study. Children 2022, 9, 1954. https://doi.org/10.3390/children9121954
Milosevic K, Malinic M, Plavec D, Lekovic Z, Lekovic A, Cobeljic M, Rsovac S. Diagnosing Single and Multiple Drug Hypersensitivity in Children: A Tertiary Care Center Retrospective Study. Children. 2022; 9(12):1954. https://doi.org/10.3390/children9121954
Chicago/Turabian StyleMilosevic, Katarina, Marija Malinic, Davor Plavec, Zoran Lekovic, Aleksa Lekovic, Mina Cobeljic, and Snezana Rsovac. 2022. "Diagnosing Single and Multiple Drug Hypersensitivity in Children: A Tertiary Care Center Retrospective Study" Children 9, no. 12: 1954. https://doi.org/10.3390/children9121954
APA StyleMilosevic, K., Malinic, M., Plavec, D., Lekovic, Z., Lekovic, A., Cobeljic, M., & Rsovac, S. (2022). Diagnosing Single and Multiple Drug Hypersensitivity in Children: A Tertiary Care Center Retrospective Study. Children, 9(12), 1954. https://doi.org/10.3390/children9121954








