Evaluation of Dysphagia and Inhalation Risk in Neurologically Impaired Children Using Esophageal High-Resolution Manometry with Swallowing Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. HRM Procedure
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Reilly, S.; Skuse, D.; Poblete, X. Prevalence of feeding problems and oral motor dysfunction in children with cerebral palsy: A community survey. J. Pediatr. 1996, 129, 877–882. [Google Scholar] [CrossRef]
- Brooks, J.; Day, S.; Shavelle, R.; Strauss, D. Low weight, morbidity and mortality in children with cerebral palsy: New clinical growth charts. Pediatrics 2011, 128, e299–e307. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. Suppl. 2007, 109, 8–14. [Google Scholar] [PubMed]
- Stevenson, R.D.; Conaway, M.; Chumlea, W.C.; Rosenbaum, P.; Fung, E.B.; Henderson, R.C.; Worley, G.; Liptak, G.; O’Donnell, M.; Samson-Fang, L.; et al. Growth and health in children with moderate-to-severe cerebral palsy. Pediatrics 2006, 118, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Samson-Fang, L.J.; Stevenson, R.D. Identification of malnutrition in children with cerebral palsy: Poor performance of weight-for-height centiles. Dev. Med. Child Neurol. 2000, 42, 162–168. [Google Scholar] [CrossRef]
- Kuperminc, M.; Stevenson, R.D. Growth and nutrition disorders in children with cerebral palsy. Dev. Disabil. Res. Rev. 2008, 14, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Fung, E.B.; Samson-Fang, L.; Stallings, V.A.; Conaway, M.; Liptak, G.; Henderson, R.C.; Worley, G.; O’donnell, M.; Calvert, R.; Rosenbaum, P.; et al. Feeding dysfunction is associated with poor growth and health status in children with cerebral palsy. J. Am. Diet. Assoc. 2002, 102, 361–373. [Google Scholar] [CrossRef]
- Marchand, V.; Motil, K.J.; Naspghan Committee on Nutrition. Nutrition support for neurologically impaired children: A clinical report of the North American Society for pediatric Gastroenterology, Hepatology, and nutrition. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Van Wynckel, M.; Hulst, J.; Broekaert, I.; Bronsky, J.; Dall’Oglio, L.; Mis, N.F.; Hojsak, I.; Orel, R.; Papadopoulou, A.; et al. European Society for Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for the Evaluation and Treatment of Gastrointestinal and Nutritional Complications in Children with Neurological Impairment. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 242–264. [Google Scholar] [CrossRef]
- Quitadamo, P.; Thapar, N.; Staiano, A.; Borrelli, O. Gastrointestinal and nutritional problems in neurologically impaired children. Eur. J. Ped. Neurol. 2016, 20, 810–815. [Google Scholar] [CrossRef]
- Zaloga, G. Aspiration-related illnesses: Definitions and diagnosis. North American summit on aspiration in the critically ill patient: Consensus statement. J. Parent. Ent. Nutr. 2002, 26, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Sorin, R.; Somers, S.; Austin, W.; Bester, S. The influence of videofluoroscopy on the management of the dysphagic patient. Dysphagia 1988, 2, 127–135. [Google Scholar] [CrossRef]
- Gomes, G.F.; Campos, A.C.; Pisani, J.C.; Macedo, E.D.; Vieira, M.C. Diagnostic methods for the detection of anterograde aspiration in enterally fed patients. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 285–292. [Google Scholar] [CrossRef] [PubMed]
- van den Engel-Hoek, L.; Erasmus, C.E.; van Hulst, K.C.; Arvedson, J.C.; de Groot, I.J.; de Swart, B.J. Children with central and peripheral neurologic disorders have distinguishable pattern of dysphagia on videofluroscopic swallow study. J. Child Neurol. 2014, 29, 646–653. [Google Scholar] [CrossRef]
- Baijens, L.; Barikroo, A.; Pilz, W. Intrarater and interrater reliability for measurements in videofluoroscopy of swallowing. Eur. J. Radiol. 2013, 82, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Leonard, R.; Rees, C.J.; Belafsky, P.; Allen, J. Fluoroscopic surrogate for pharyngeal strength: The pharyngeal constriction ratio (PCR). Dysphagia 2011, 26, 13–17. [Google Scholar] [CrossRef]
- Takasaki, K.; Umeki, H.; Enatsu, K.; Tanaka, F.; Sakihama, N.; Kumagami, H.; Takahashi, H. Investigation of pharyngeal swallowing function using high-resolution manometry. Laryngoscope 2008, 118, 1729–1732. [Google Scholar] [CrossRef]
- Roman, S.; Pandolfino, J.; Mion, F. High-resolution manometry: A new gold standard to diagnose esophageal dysmotility? Gastroenterol. Clin. Biol. 2009, 33, 1061–1067. [Google Scholar] [CrossRef]
- Park, D.; Oh, Y.; Ryu, J.S. Findings of abnormal videofluoroscopic swallowing study identified by high-resolution manometry parameters. Arch. Phys. Med. Rehabil. 2016, 97, 421–428. [Google Scholar] [CrossRef]
- Savarino, E.; De Bortoli, N.; Bellini, M.; Galeazzi, F.; Ribolsi, M.; Salvador, R.; Savarino, V.; Penagini, R. Practice guidelines on the use of esophageal manometry—A gismad-sige-aigo medical position statement. Dig. Liver Dis. 2016, 48, 1124–1135. [Google Scholar] [CrossRef]
- Yoon, K.J.; Park, J.H.; Park, J.H.; Jung, I.S. Videofluoroscopic and manometric evaluation of pharyngeal and upper esophageal sphincter function during swallowing. J. Neurogastroenterol. Motil. 2014, 20, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Kim, D.K.; Lee, Y.T.; Yi, Y.; Lee, J.S.; Kim, K.; Park, J.H.; Yoon, K.J. Quantitative Analysis of Swallowing Function Between Dysphagia Patients and Healthy Subjects Using High-Resolution Manometry. Ann. Rehabil. Med. 2017, 41, 776–785. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Castell, J.A.; Castell, D.O. Modern solid state computerized manometry of the pharyngoesophageal segment. Dysphagia 1993, 8, 270–275. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, T.M.; Hoffman, M.R.; Ciucci, M.R. High-resolution manometry of pharyngeal swallow pressure events associated with head turn and chin tuck. Ann. Otol. Rhinol. Laryngol. 2010, 119, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Kahrilas, P.J.; Dodds, W.J.; Dent, J.; Logemann, J.A.; Shaker, R. Upper esophageal sphincter function during deglutition. Gastroenterology 1988, 95, 52–62. [Google Scholar] [CrossRef]
- Costa, M.M.B. Neural control of swallowing. Arq. Gastroenterol. 2018, 55, 61–75. [Google Scholar] [CrossRef]
- Matsuo, K.; Palmer, J.B. Anatomy and Physiology of Feeding and Swallowing: Normal and Abnormal. Phys. Med. Rehabil. Clin. N. Am. 2008, 19, 691–707. [Google Scholar] [CrossRef]
- Panebianco, M.; Marchese-Ragona, R.; Masiero, S.; Restivo, D.A. Dysphagia in neurological diseases: A literature review. Neurol. Sci. 2020, 41, 3067–3073. [Google Scholar] [CrossRef]
- Seddon, P.C.; Khan, Y. Respiratory problems in children with neurological impairment. Arch. Dis. Child. 2003, 88, 75–78. [Google Scholar] [CrossRef]
- Sivarao, D.V.; Goyal, R.K. Functional Anatomy and Physiology of the Upper Esophageal Sphincter. Am. J. Med. 2000, 108, 27–37. [Google Scholar] [CrossRef]
- Omari, T.; Schar, M. High-resolution manometry: What about the pharynx? Curr. Opin. Otolaryngol. Head Neck Surg. 2018, 26, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, E.; Di Piero, V. Dysphagia: Pathophysiology, diagnosis and treatment. Front. Neurol. Neurosci. 2012, 30, 86–89. [Google Scholar] [CrossRef] [PubMed]
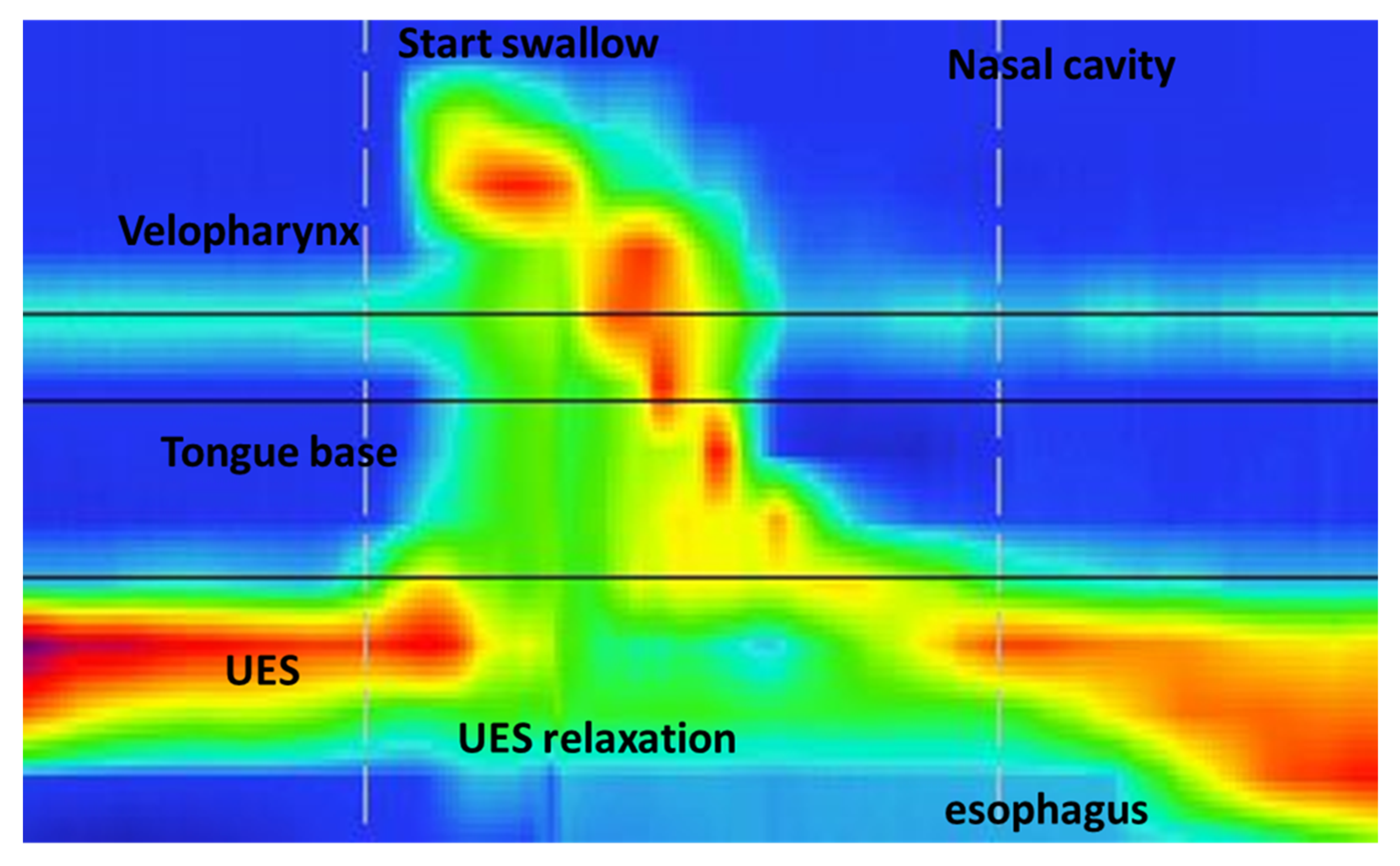
| Characteristic | NI Children (25) | Control Group (25) |
|---|---|---|
| Age (months) | 105.48 ± 53.11 | 124.8 ± 47.25 |
| Gender | 12 M/13 F | 10 M/15 F |
| BMI | 13.36 (r 6–27) | 20.45 (15–26) |
| HRM Parameters | NI Group Mean (± SD) | Control Group Mean (± SD) | p-Value |
|---|---|---|---|
| VP maximal pressure (mmHg) | 120.96 ± 21.26 | 212.72 ± 42.46 | <0.0005 |
| VP duration (s) | 0.61 ± 0.15 | 0.91± 0.27 | <0.0005 |
| TB maximal pressure (mmHg) | 144.44 ± 53.43 | 243.84 ± 28.56 | <0.0005 |
| TB duration (s) | 0.48 ± 0.11 | 0.68 ± 0.17 | <0.0005 |
| UES maximal pressure (mmHg) | 251.08 ± 47.32 | 284.12 ± 36.01 | 0.008 |
| UES minimal pressure (mmHg) | 6.69 ± 2.79 | 1.49 ± 2.93 | <0.0005 |
| UES relaxation duration (s) | 0.42 ± 0.10 | 0.74 ± 0.12 | <0.0005 |
| UES resting pressure (mmHg) | 35.52 ± 20.24 | 103.32 ± 11.50 | <0.0005 |
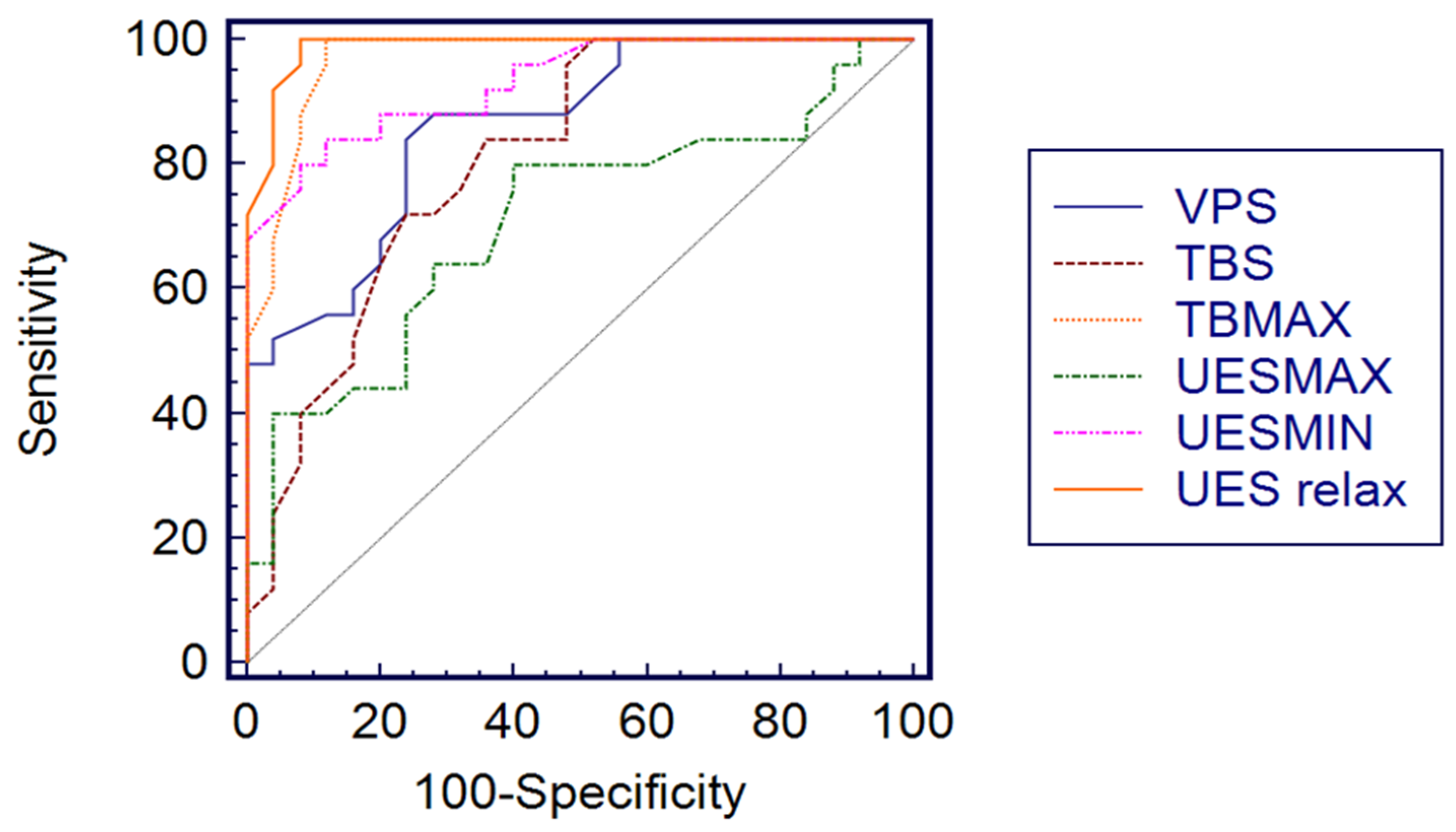
| HRM Variable | Optimal Threshold Value | AUC (95% CI) | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | +LR | −LR |
|---|---|---|---|---|---|---|
| VP p max | 155 | 1.00 (0.928–1.00) | 100 (86.2–100) | 100 (86.2–100) | 0.00 | |
| VP s | 0.76 | 0.86 (0.734–0.943) | 84 (63.9–95.4) | 76 (50.6–87.9) | 3.50 | 0.21 |
| TB p max | 205 | 0.970 (0.877–0.996) | 100 (86.2–100) | 88 (68.8–97.3) | 8.33 | 0.00 |
| TB s | 0.52 | 0.811 (0.675–0.908) | 72 (50.6–87.9) | 76 (54.9–90.6) | 3.00 | 0.37 |
| UES p max | 285 | 0.707 (0.561–0.827) | 80 (59.3–93.1) | 60 (38.7–78.8) | 2.00 | 0.33 |
| UES p min | 4.3 | 0.931 (0.822–0.983) | 80 (59.3–93.1) | 92 (73.9–98.8) | 10.00 | 0.22 |
| UES relax | 0.6 | 0.988 (0.906–0.995) | 100 (86.2–100) | 92 (73.9–98.8) | 12.5 | 0.00 |
| UES rest | 65 | 1.00 (0.928–1.00) | 100 (86.2–100) | 100 (86.2–100) | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caruso, A.M.; Bommarito, D.; Girgenti, V.; Amato, G.; Figuccia, A.; Casuccio, A.; Ferlisi, A.; Genuardi, R.; La Fata, S.; Mattei, R.; et al. Evaluation of Dysphagia and Inhalation Risk in Neurologically Impaired Children Using Esophageal High-Resolution Manometry with Swallowing Analysis. Children 2022, 9, 1987. https://doi.org/10.3390/children9121987
Caruso AM, Bommarito D, Girgenti V, Amato G, Figuccia A, Casuccio A, Ferlisi A, Genuardi R, La Fata S, Mattei R, et al. Evaluation of Dysphagia and Inhalation Risk in Neurologically Impaired Children Using Esophageal High-Resolution Manometry with Swallowing Analysis. Children. 2022; 9(12):1987. https://doi.org/10.3390/children9121987
Chicago/Turabian StyleCaruso, Anna Maria, Denisia Bommarito, Vincenza Girgenti, Glenda Amato, Adele Figuccia, Alessandra Casuccio, Annalisa Ferlisi, Rosaria Genuardi, Sabrina La Fata, Rosalia Mattei, and et al. 2022. "Evaluation of Dysphagia and Inhalation Risk in Neurologically Impaired Children Using Esophageal High-Resolution Manometry with Swallowing Analysis" Children 9, no. 12: 1987. https://doi.org/10.3390/children9121987
APA StyleCaruso, A. M., Bommarito, D., Girgenti, V., Amato, G., Figuccia, A., Casuccio, A., Ferlisi, A., Genuardi, R., La Fata, S., Mattei, R., Milazzo, M. P. M., & Di Pace, M. R. (2022). Evaluation of Dysphagia and Inhalation Risk in Neurologically Impaired Children Using Esophageal High-Resolution Manometry with Swallowing Analysis. Children, 9(12), 1987. https://doi.org/10.3390/children9121987







