Association of Consuming Tap Water or Purified Water during Infancy with Irritable Bowel Syndrome in Children
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Data Sources
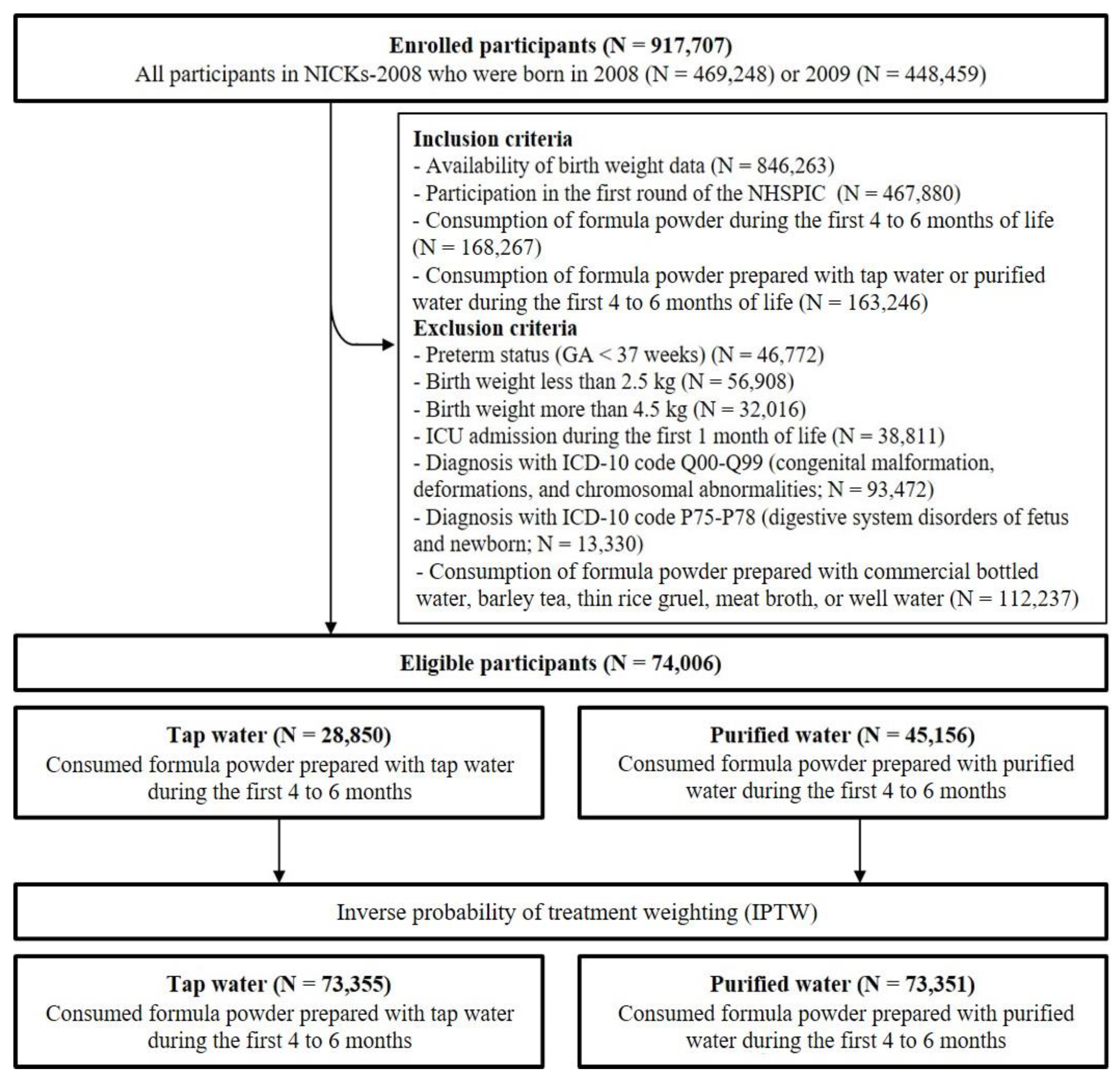
2.3. Participants
2.4. Exposure
2.5. Outcomes
2.6. Covariates
2.7. Statistical Analysis
3. Results
3.1. Participants
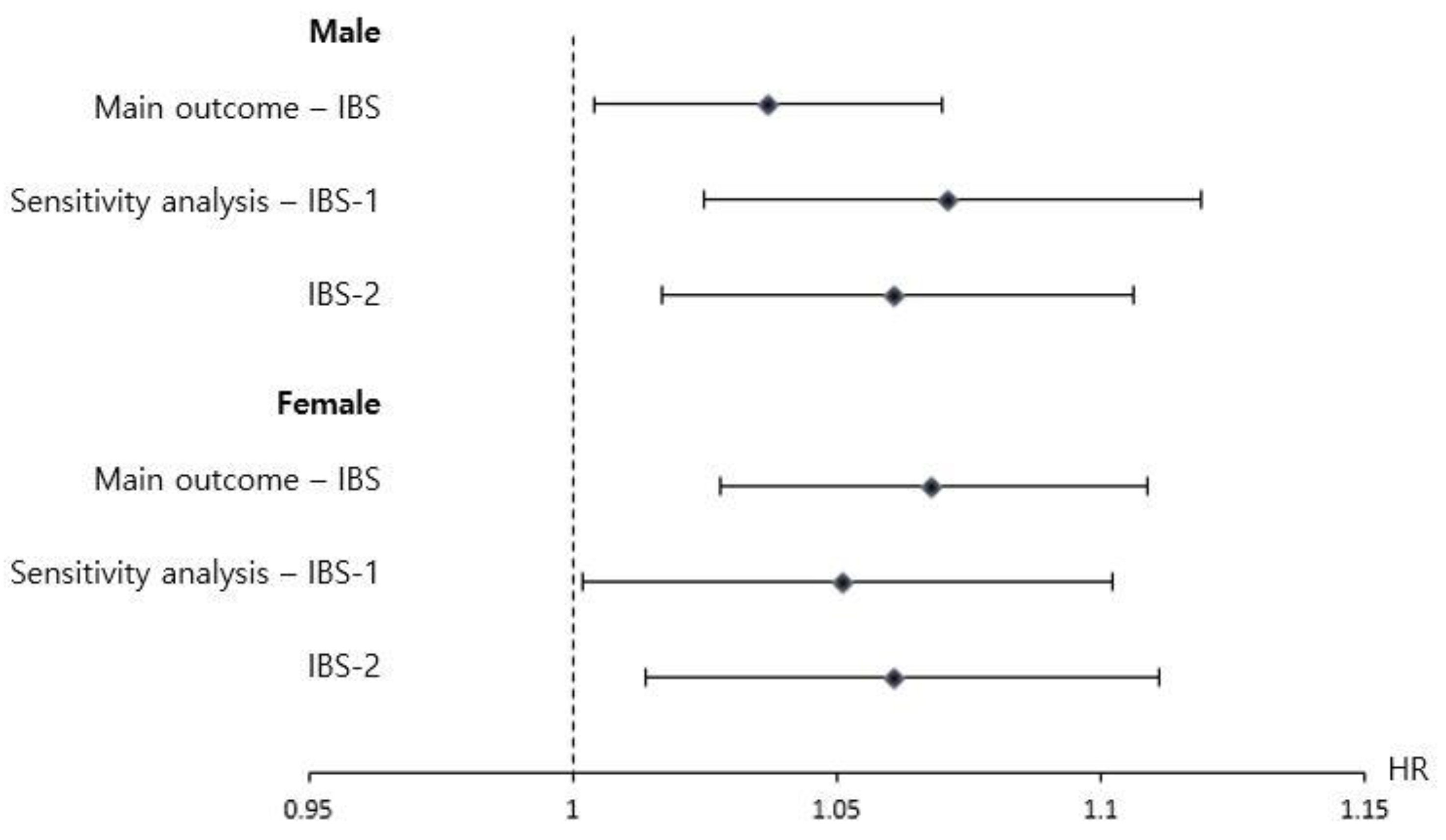
3.2. Association of the Type of Drinking Water with a Diagnosis of IBS after 4 Years of Age
3.3. Sensitivity Analysis
3.4. Post Hoc Analysis: Tap Water vs. Commercial Bottled Water
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Fawell, J.; Nieuwenhuijsen, M.J. Contaminants in drinking waterEnvironmental pollution and health. Br. Med. Bull. 2003, 68, 199–208. [Google Scholar] [CrossRef]
- Ashbolt, N.J. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology 2004, 198, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.M.; Summers, R.; Plummer, R. Cisterns and safe drinking water in Canada. Can. Water Resour. J. 2013, 38, 121–134. [Google Scholar] [CrossRef]
- Jeong, S. Factors influencing development of the infant microbiota: From prenatal period to early infancy. Clin. Exp. Pediatr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zeissig, S.; Blumberg, R.S. Life at the beginning: Perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nat. Immunol. 2014, 15, 307–310. [Google Scholar] [CrossRef]
- Gerber, J.S.; Bryan, M.; Ross, R.K.; Daymont, C.; Parks, E.P.; Localio, A.R.; Grundmeier, R.W.; Stallings, V.A.; Zaoutis, T.E. Antibiotic exposure during the first 6 months of life and weight gain during childhood. Jama 2016, 315, 1258–1265. [Google Scholar] [CrossRef]
- Lee, J.; Lee, C.; Hugunin, K.; Maute, C.; Dysko, R. Bacteria from drinking water supply and their fate in gastrointestinal tracts of germ-free mice: A phylogenetic comparison study. Water Res. 2010, 44, 5050–5058. [Google Scholar] [CrossRef]
- Bowyer, R.C.; Schillereff, D.N.; Jackson, M.A.; Le Roy, C.; Wells, P.M.; Spector, T.D.; Steves, C.J. Associations between UK tap water and gut microbiota composition suggest the gut microbiome as a potential mediator of health differences linked to water quality. Sci. Total Environ. 2020, 739, 139697. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.E.; Shim, S.M.; Ha, E.K.; Yon, D.K.; Kim, O.H.; Baek, J.H.; Koh, H.Y.; Chae, K.Y.; Lee, S.W. Cohort profile: National Investigation of Birth Cohort in Korea study 2008 (NICKs-2008). Clin. Exp. Pediatrics 2021, 64, 480–488. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.W.; Lee, J.E.; Ha, E.K.; Han, M.Y.; Lee, E. Breastmilk feeding during the first 4 to 6 months of age and childhood disease burden until 10 years of age. Nutrients 2021, 13, 2825. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.-K.; Chen, A.-C.; Lin, C.-L.; Shen, T.-C.; Li, T.-C.; Wei, C.-C. Preschoolers with allergic diseases have an increased risk of irritable bowel syndrome when reaching school age. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Goff, S.L.; Feld, A.; Andrade, S.E.; Mahoney, L.; Beaton, S.J.; Boudreau, D.M.; Davis, R.L.; Goodman, M.; Hartsfield, C.L.; Platt, R. Administrative data used to identify patients with irritable bowel syndrome. J. Clin. Epidemiol. 2008, 61, 617–621. [Google Scholar] [CrossRef]
- Sato, T.; Matsuyama, Y. Marginal structural models as a tool for standardization. Epidemiology 2003, 14, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Brookhart, M.A.; Wyss, R.; Layton, J.B.; Stürmer, T. Propensity score methods for confounding control in nonexperimental research. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 604–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Lee, B.J.; Bak, Y.-T. Irritable bowel syndrome, gut microbiota and probiotics. J. Neurogastroenterol. Motil. 2011, 17, 252. [Google Scholar] [CrossRef] [Green Version]
- Isolauri, E. Development of healthy gut microbiota early in life. J. Paediatr. Child Health 2012, 48, 1–6. [Google Scholar] [CrossRef]
- Dias, M.F.; Reis, M.P.; Acurcio, L.B.; Carmo, A.O.; Diamantino, C.F.; Motta, A.M.; Kalapothakis, E.; Nicoli, J.R.; Nascimento, A.M. Changes in mouse gut bacterial community in response to different types of drinking water. Water Res. 2018, 132, 79–89. [Google Scholar] [CrossRef]
- Chitkara, D.K.; van Tilburg, M.A.; Blois-Martin, N.; Whitehead, W.E. Early life risk factors that contribute to irritable bowel syndrome in adults: A systematic review. Am. J. Gastroenterol. 2008, 103, 765–774. [Google Scholar] [CrossRef] [Green Version]
- Lydiard, R.B. Irritable bowel syndrome, anxiety, and depression: What are the links? J. Clin. Psychiatry 2001, 62, 38–47. [Google Scholar]
- Waehrens, R.; Li, X.; Sundquist, J.; Sundquist, K.; Zöller, B. Perinatal and familial risk factors for irritable bowel syndrome in a Swedish national cohort. Scand. J. Gastroenterol. 2018, 53, 559–566. [Google Scholar] [CrossRef] [PubMed]
| Sociodemographic Characteristics | Observed Data (N = 74,006) | Weighted Data (N = 146,706) b | ||||
|---|---|---|---|---|---|---|
| N (%) c | Standardized Difference (%) f | N (%) c | Standardized Difference (%) d | |||
| Tap Water d | Purified Water e | Tap Water d | Purified Water e | |||
| (N = 28,850) | (N = 45,156) | (N = 73,355) | (N = 73,351) | |||
| Sex | ||||||
| Male | 15,156 (52.5) | 23,704 (52.5) | 0.1 | 38,532 (52.5) | 38,506 (52.5) | 0.1 |
| Female | 13,694 (47.5) | 21,452 (47.5) | 34,824 (47.5) | 34,845 (47.5) | ||
| Residence at birth g | ||||||
| Seoul | 6625 (23.0) | 10,615 (23.5) | 1.3 | 17,115 (23.3) | 17,089 (23.3) | 0.0 |
| Metropolitan | 6732 (23.3) | 11,165 (24.7) | 3.3 | 17,715 (24.2) | 17,737 (24.2) | 0.1 |
| Urban | 11,614 (40.3) | 18,361 (40.7) | 0.8 | 29,715 (40.5) | 29,712 (40.5) | 0.1 |
| Rural | 3619 (12.5) | 4620 (10.2) | 7.3 | 8162 (11.1) | 8164 (11.1) | 0.0 |
| Birth weight, kg (SD) h | 3.22 (0.32) | 3.22 (0.32) | 1.5 | 3.22 (0.51) | 3.22 (0.41) | 0.0 |
| Income quintile i | ||||||
| 1 (Lowest) | 2300 (8.0) | 3384 (7.5) | 1.8 | 5643 (7.7) | 5636 (7.7) | 0.0 |
| 2 | 4557 (15.8) | 6177 (13.7) | 6.0 | 10,628 (14.5) | 10,632 (14.5) | 0.0 |
| 3 (Middle) | 8215 (28.5) | 11,238 (24.5) | 8.1 | 19,316 (26.3) | 19,305 (26.3) | 0.0 |
| 4 | 9141 (31.7) | 14,374 (31.8) | 0.3 | 23,287 (31.7) | 23,297 (31.8) | 0.0 |
| 5 (Highest) | 3757 (13.0) | 8399 (18.6) | 15.3 | 12,044 (16.4) | 12,042 (16.4) | 0.0 |
| Birth year | ||||||
| 2008 | 13,140 (45.5) | 21,566 (47.8) | 4.4 | 34,291 (46.7) | 34,318 (46.8) | 0.1 |
| 2009 | 15,710 (54.5) | 23,590 (52.2) | 39,064 (53.3) | 39,033 (53.2) | ||
| Clinical Characteristics | Observed Data (N = 74,006) | Weighted Data (N = 146,706) b | ||||
|---|---|---|---|---|---|---|
| N (%) c | Standardized Difference (%) f | N (%) c | Standardized Difference (%) f | |||
| Tap Water d | Purified Water e | Tap Water d | Purified Water e | |||
| (N = 28,850) | (N = 45,156) | (N = 73,355) | (N = 73,351) | |||
| Hospital utilization within six months of age | ||||||
| Visiting to pediatricians | 28,178 (97.7) | 44,116 (97.7) | 0.2 | 71,666 (97.7) | 71,658 (97.7) | 0.0 |
| Visiting ER | 2281 (7.9) | 3789 (8.4) | 1.7 | 6030 (8.2) | 6010 (8.2) | 0.0 |
| Hospitalization | 5150 (17.9) | 8419 (18.6) | 2.1 | 13,452 (18.3) | 13,458 (18.3) | 0.0 |
| Certain conditions (ICD-10 codes) originating in the perinatal period | ||||||
| Respiratory and cardiovascular disorders specific to the perinatal period | 1199 (4.2) | 1793 (4.0) | 0.9 | 2974 (4.1) | 2967 (4.1) | 0.0 |
| Infections specific to the perinatal period | 4066 (14.1) | 6176 (13.7) | 1.2 | 10,152 (13.8) | 10,146 (13.8) | 0.0 |
| Hemorrhagic and hematological disorders of fetuses and newborns | 8051 (27.9) | 12,608 (27.9) | 0.0 | 20,483 (27.9) | 20,484 (27.9) | 0.0 |
| Prevalent diseases (ICD-10 codes) diagnosed within six months of age | ||||||
| Viral intestinal infection, unspecified | 9741 (2.7) | 459 (5.2) | 12.9 | 10,064 (2.8) | 11,772 (3.3) | 0.6 |
| Other and unspecified gastroenteritis and colitis of infectious origin | 18,362 (5.1) | 707 (8.0) | 11.9 | 18,802 (5.2) | 20,941 (5.8) | 0.7 |
| Gastroenteritis and colitis of unspecified origin | 20,055 (5.6) | 781 (8.9) | 12.8 | 20,541 (5.7) | 22,505 (6.3) | 0.8 |
| Conjunctivitis, unspecified | 14,776 (4.1) | 397 (4.5) | 2.0 | 14,961 (4.1) | 14,662 (4.1) | 0.4 |
| Otitis media, unspecified | 7989 (2.2) | 204 (2.3) | 0.7 | 8058 (2.2) | 7869 (2.2) | 0.2 |
| Acute nasopharyngitis | 104,381 (29.0) | 3,056 (34.8) | 12.4 | 105,974 (29.2) | 107,273 (29.8) | 0.1 |
| Acute sinusitis, unspecified | 12,585 (3.5) | 304 (3.5) | 0.2 | 12,707 (3.5) | 12,261 (3.4) | 0.4 |
| Acute pharyngitis | 37,920 (10.5) | 1103 (12.6) | 6.3 | 38,483 (10.6) | 39,901 (11.1) | 0.6 |
| Acute tonsillitis, unspecified | 18,232 (5.1) | 464 (5.3) | 1.0 | 18,411 (5.1) | 18,064 (5.0) | 0.1 |
| Other acute upper respiratory infections of multiple sites | 9292 (2.6) | 265 (3.0) | 2.6 | 9427 (2.6) | 9916 (2.8) | 0.3 |
| Acute upper respiratory infection, unspecified | 81,665 (22.7) | 2198 (25.0) | 5.4 | 82,711 (22.8) | 84,377 (23.4) | 0.5 |
| Pneumonia, unspecified | 9496 (2.6) | 225 (2.6) | 0.5 | 9569 (2.6) | 10,044 (2.8) | 0.6 |
| Acute bronchitis | 66,304 (18.4) | 1,634 (18.6) | 0.4 | 66,995 (18.4) | 66,559 (18.5) | 0.7 |
| Acute bronchiolitis | 48,798 (13.6) | 1,129 (12.8) | 2.1 | 49,176 (13.5) | 50,601 (14.1) | 0.7 |
| Gastro-esophageal reflux disease without esophagitis | 8416 (2.3) | 333 (3.8) | 8.4 | 8617 (2.4) | 8590 (2.3) | 0.4 |
| Functional dyspepsia | 7635 (2.1) | 315 (3.6) | 8.8 | 7834 (2.2) | 8289 (5.1) | 0.2 |
| Noninfective gastroenteritis and colitis, unspecified | 16,372 (4.6) | 561 (6.4) | 8.1 | 16,709 (4.6) | 18,306 (5.1) | 0.8 |
| Constipation | 15,365 (4.3) | 574 (6.5) | 10.0 | 15,699 (4.3) | 16,272 (4.5) | 0.7 |
| Functional diarrhea | 5223 (1.5) | 205 (2.3) | 6.5 | 5360 (1.5) | 5375 (1.5) | 0.0 |
| Functional intestinal disorder, unspecified | 10,060 (2.8) | 357 (4.1) | 7.0 | 10,248 (2.8) | 10,274 (2.9) | 0.1 |
| Nausea and vomiting | 10,334 (2.9) | 495 (5.6) | 13.7 | 10,699 (2.9) | 11,869 (3.3) | 0.5 |
| Fever, unspecified | 18,237 (5.1) | 524 (6.0) | 3.9 | 18,507 (5.1) | 21,194 (5.9) | 0.1 |
| Drug (drug classification code) used within six months of age | ||||||
| Antipyretics | 17,166 (59.5) | 28,027 (62.1) | 5.3 | 44,808 (61.1) | 44,796 (61.1) | 0.0 |
| Antihistamine | 19,707 (68.3) | 32,541 (72.1) | 8.2 | 51,843 (70.7) | 51,817 (70.6) | 0.0 |
| Digestive anti-ulcer drug | 524 (1.8) | 884 (2.0) | 1.0 | 1384 (1.9) | 1390 (1.9) | 0.0 |
| Antiacid drug | 313 (1.1) | 558 (1.2) | 1.4 | 858 (1.2) | 861 (1.2) | 0.0 |
| Probiotics | 20,061 (69.5) | 32,396 (71.7) | 4.8 | 51,976 (70.9) | 51,983 (70.2) | 0.0 |
| Laxative drug | 39 (0.1) | 76 (0.2) | 0.9 | 114 (0.2) | 114 (0.2) | 0.0 |
| Other digestive system drugs | 11,080 (38.4) | 17,939 (39.7) | 2.7 | 28,760 (39.2) | 28,760 (39.2) | 0.0 |
| Steroid | 3481 (12.1) | 6,199 (13.7) | 5.0 | 9588 (13.1) | 9592 (13.1) | 0.0 |
| Observed Data (N = 74,006) | Weighted Data (N = 146,706) b | HR (95% CI) e | p-Value | |||
|---|---|---|---|---|---|---|
| N (%) | N (%) | |||||
| Tap water c | Purified water d | Tap water c | Purified water d | |||
| (N = 28,850) | (N = 45,156) | (N = 73,355) | (N = 73,351) | |||
| Main outcome | ||||||
| IBS f | 4380 (15.2) | 7216 (16.0) | 11,197 (15.3) | 11,721 (16.0) | 1.051 (1.013 to 1.090) | <0.01 |
| Sensitivity analysis | ||||||
| IBS-1 g | 2787 (9.66) | 4686 (10.4) | 7176 (9.8) | 7598 (10.4) | 1.062 (1.014 to 1.111) | 0.01 |
| IBS-2 h | 3059 (10.6) | 5103 (11.3) | 7827 (10.7) | 8277 (11.3) | 1.061 (1.016 to 1.108) | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Baek, H.-S.; Ha, E.K.; Cha, H.R.; Lee, S.W.; Han, M.Y. Association of Consuming Tap Water or Purified Water during Infancy with Irritable Bowel Syndrome in Children. Children 2022, 9, 135. https://doi.org/10.3390/children9020135
Kim JH, Baek H-S, Ha EK, Cha HR, Lee SW, Han MY. Association of Consuming Tap Water or Purified Water during Infancy with Irritable Bowel Syndrome in Children. Children. 2022; 9(2):135. https://doi.org/10.3390/children9020135
Chicago/Turabian StyleKim, Ju Hee, Hey-Sung Baek, Eun Kyo Ha, Hye Ryung Cha, Seung Won Lee, and Man Yong Han. 2022. "Association of Consuming Tap Water or Purified Water during Infancy with Irritable Bowel Syndrome in Children" Children 9, no. 2: 135. https://doi.org/10.3390/children9020135







