Utility of Three-Dimensional Printed Model in Biventricular Repair of Complex Congenital Cardiac Defects: Case Report and Review of Literature
Abstract
:1. Introduction
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Serraf, A.; Bensari, N.; Houyel, L.; Capderou, A.; Roussin, R.; Lebret, E.; Ly, M.; Belli, E. Surgical management of congenital heart defects associated with heterotaxy syndrome. Eur. J. Cardiothorac. Surg. 2010, 38, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Makhija, Z.; Marwah, A.; Mishra, S.; Kumar, J.; Goel, A.; Sharma, R. Biventricular repair in heterotaxy patients. World J. Pediatr. Congenit. Heart Surg. 2015, 6, 195–202. [Google Scholar] [CrossRef] [PubMed]
- McGovern, E.; Kelleher, E.; Potts, J.E.; O’Brien, J.; Walsh, K.; Nolke, L.; McMahon, C.J. Predictors of poor outcome among children with heterotaxy syndrome: A retrospective review. Open Heart 2016, 3, e000328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lively-Endicott, H.; Lara, D.A. Successful Palliation via Kawashima Procedure of an Infant with Heterotaxy Syndrome and Left-Atrial Isomerism. Ochsner. J. 2018, 18, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.G.; Bacha, E.A.; Marx, G.R.; Marshall, A.; Fynn-Thompson, F.; Mayer, J.E.; Del Nido, P.; Pigula, F.A. Biventricular repair in patients with heterotaxy syndrome. J. Thorac. Cardiovasc. Surg. 2009, 137, 371–379.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betancourt, L.A.G.; Singh, H.R.; Agarwal, A. Partial Anomalous Left Pulmonary Artery in Heterotaxy Syndrome. A Case Report and Review of Literature. Prog. Pediatric Cardiol. 2020, 60, 101304. [Google Scholar] [CrossRef]
- Gilljam, T.; McCrindle, B.W.; Smallhorn, J.F.; Williams, W.G.; Freedom, R.M. Outcomes of left atrial isomerism over a 28-year period at a single institution. J. Am. Coll. Cardiol. 2000, 36, 908–916. [Google Scholar] [CrossRef] [Green Version]
- Hashmi, A.; Abu-Sulaiman, R.; McCrindle, B.W.; Smallhorn, J.F.; Williams, W.G.; Freedom, R.M. Management and outcomes of right atrial isomerism: A 26-year experience. J. Am. Coll. Cardiol. 1998, 31, 1120–1126. [Google Scholar] [CrossRef] [Green Version]
- Sinzobahamvya, N.; Arenz, C.; Brecher, A.M.; Urban, A.E. Atrial isomerism: A surgical experience. Cardiovasc. Surg. 1999, 7, 436–442. [Google Scholar] [CrossRef]
- Cheung, Y.F.; Cheng, V.Y.; Chau, A.K.; Chiu, C.S.; Yung, T.C.; Leung, M.P. Outcome of infants with right atrial isomerism: Is prognosis better with normal pulmonary venous drainage? Heart 2002, 87, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Heinemann, M.K.; Hanley, F.L.; Van Praagh, S.; Fenton, K.N.; Jonas, R.A.; Mayer, J.E., Jr.; Castaneda, A.R. Total anomalous pulmonary venous drainage in newborns with visceral heterotaxy. Ann. Thorac. Surg. 1994, 57, 88–91. [Google Scholar] [CrossRef]
- Cohen, M.S.; Schultz, A.H.; Tian, Z.Y.; Donaghue, D.D.; Weinberg, P.M.; Gaynor, J.W.; Rychik, J. Heterotaxy syndrome with functional single ventricle: Does prenatal diagnosis improve survival? Ann. Thorac. Surg. 2006, 82, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Bartz, P.J.; Driscoll, D.J.; Dearani, J.A.; Puga, F.J.; Danielson, G.K.; O’Leary, P.W.; Earing, M.G.; Warnes, C.A.; Hodge, D.O.; Cetta, F. Early and late results of the modified fontan operation for heterotaxy syndrome 30 years of experience in 142 patients. J. Am. Coll. Cardiol. 2006, 48, 2301–2305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirooka, K.; Yagihara, T.; Kishimoto, H.; Isobe, F.; Yamamoto, F.; Nishigaki, K.; Matsuki, O.; Uemura, H.; Kawashima, Y. Biventricular repair in cardiac isomerism. Report of seventeen cases. J. Thorac. Cardiovasc. Surg. 1995, 109, 530–535. [Google Scholar] [CrossRef] [Green Version]
- Koh, M.; Yagihara, T.; Uemura, H.; Kagisaki, K.; Hagino, I.; Ishizaka, T.; Kitamura, S. Biventricular repair for right atrial isomerism. Ann. Thorac. Surg. 2006, 81, 1808–1816, discussion 1816. [Google Scholar] [CrossRef]
- De Moraes, P.H.; Olate, S.; Cantín, M.; Assis, A.F.; Santos, E.; Silva, F.O.; Silva, L.O. Anatomical reproducibility through 3D printing in cranio-maxillo-facial defects. Int. J. Morphol. 2015, 33, 826–830. [Google Scholar] [CrossRef] [Green Version]
- Darwood, A.; Collier, J.; Joshi, N.; Grant, W.E.; Sauret-Jackson, V.; Richards, R.; Dawood, A.; Kirkpatrick, N. Re-thinking 3D printing: A novel approach to guided facial contouring. J. Cranio-Maxillofac. Surg. 2015, 43, 1256–1260. [Google Scholar] [CrossRef]
- Liu, Z.; Jia, J.; Zhang, Y.; Tian, W.; Jin, X.; Hu, Y. Internal fixation of complicated acetabular fractures directed by preoperative surgery with 3D printing models. Orthop. Surg. 2017, 9, 257–260. [Google Scholar] [CrossRef] [Green Version]
- Riesenkampff, E.; Rietdorf, U.; Wolf, I.; Schnackenburg, B.; Ewert, P.; Huebler, M.; Alexi-Meskishvili, V.; Anderson, R.H.; Engel, N.; Meinzer, H.P.; et al. The practical clinical value of three-dimensional models of complex congenitally malformed hearts. J. Thorac. Cardiovasc. Surg. 2009, 138, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Farooqi, K.M.; Gonzalez-Lengua, C.; Shenoy, R.; Sanz, J.; Nguyen, K. Use of a Three Dimensional Printed Cardiac Model to Assess Suitability for Biventricular Repair. World J. Pediatr. Congenit. Heart Surg. 2016, 7, 414–416. [Google Scholar] [CrossRef]
- Garekar, S.; Bharati, A.; Chokhandre, M.; Mali, S.; Trivedi, B.; Changela, V.P.; Solanki, N.; Gaikwad, S.; Agarwal, V. Clinical Application and Multidisciplinary Assessment of Three Dimensional Printing in Double Outlet Right Ventricle With Remote Ventricular Septal Defect. World J. Pediatr. Congenit. Heart Surg. 2016, 7, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Kappanayil, M.; Koneti, N.R.; Kannan, R.R.; Kottayil, B.P.; Kumar, K. Three-dimensional-printed cardiac prototypes aid surgical decision-making and preoperative planning in selected cases of complex congenital heart diseases: Early experience and proof of concept in a resource-limited environment. Ann. Pediatr. Cardiol. 2017, 10, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Singh, G.K.; Varughese, J.; Nguyen, H.; Billadello, J.J.; Sheybani, E.F.; Woodard, P.K.; Manning, P.; Eghtesady, P. 3D Printing in Complex Congenital Heart Disease: Across a Spectrum of Age, Pathology, and Imaging Techniques. JACC Cardiovasc. Imaging 2017, 10, 953–956. [Google Scholar] [CrossRef]
- Bhatla, P.; Tretter, J.T.; Chikkabyrappa, S.; Chakravarti, S.; Mosca, R.S. Surgical planning for a complex double-outlet right ventricle using 3D printing. Echocardiography 2017, 34, 802–804. [Google Scholar] [CrossRef]
- Hoashi, T.; Ichikawa, H.; Nakata, T.; Shimada, M.; Ozawa, H.; Higashida, A.; Kurosaki, K.; Kanzaki, S.; Shiraishi, I. Utility of a super-flexible three-dimensional printed heart model in congenital heart surgery. Interact. Cardiovasc. Thorac. Surg. 2018, 27, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Vettukattil, J.J.; Mohammad Nijres, B.; Gosnell, J.M.; Samuel, B.P.; Haw, M.P. Three-dimensional printing for surgical planning in complex congenital heart disease. J. Card. Surg. 2019, 34, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A. Three-Dimensional printed cardiac model in assisting surgical planning in Heterotaxy patient with complex systemic and pulmonary venous drainage. In Cardiovascular Magnetic Resonance; Barcelona, Spain, 2018. [Google Scholar]
- Valverde, I.; Gomez-Ciriza, G.; Hussain, T.; Suarez-Mejias, C.; Velasco-Forte, M.N.; Byrne, N.; Ordonez, A.; Gonzalez-Calle, A.; Anderson, D.; Hazekamp, M.G.; et al. Three-dimensional printed models for surgical planning of complex congenital heart defects: An international multicentre study. Eur. J. Cardiothorac. Surg. 2017, 52, 1139–1148. [Google Scholar] [CrossRef]
- Milano, E.G.; Capelli, C.; Wray, J.; Biffi, B.; Layton, S.; Lee, M.; Caputp, M.; Taylor, A.M.; Schievano, S.; Biglino, G. Current and future applications of 3D printing in congenital cardiology and cardiac surgery. Br. J. Radiol. 2019, 92, 20180389. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.-J.; Thabit, O.; Kim, E.K.; Ide, H.; Y, D.; Dragulescu, A.; Seed, M.; Grosse-Wortmann, L.; van Arsdell, G. 3D printing in medicine of congenital heart diseases. 3d Print. Med. 2016, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Valverde, I.; Gomez, G.; Gonzalez, A.; Suarez-Mejias, C.; Adsuar, A.; Coserria, J.F.; Uribe, S.; Gomez-Cia, T.; Hosseinpour, A.R. Three-dimensional patient-specific cardiac model for surgical planning in Nikaidoh procedure. Cardiol. Young 2015, 25, 698–704. [Google Scholar] [CrossRef]
- Bhatla, P.; Tretter, J.T.; Ludomirsky, A.; Argilla, M.; Latson, L.A., Jr.; Chakravarti, S.; Barker, P.C.; Yoo, S.J.; McElhinney, D.B.; Wake, N.; et al. Utility and Scope of Rapid Prototyping in Patients with Complex Muscular Ventricular Septal Defects or Double-Outlet Right Ventricle: Does it Alter Management Decisions? Pediatr. Cardiol. 2017, 38, 103–114. [Google Scholar] [CrossRef] [PubMed]
- McMenamin, P.G.; Quayle, M.R.; McHenry, C.R.; Adams, J.W. The production of anatomical teaching resources using three-dimensional (3D) printing technology. Anat. Sci. Educ. 2014, 7, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Li, K.H.C.; Kui, C.; Lee, E.K.M.; Ho, C.S.; Sunny Hei, S.H.; Wu, W.; Wong, W.T.; Voll, J.; Li, G.; Liu, T. The role of 3D printing in anatomy education and surgical training: A narrative review. MedEdPublish 2017, 6, 92. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.B.; Sung, R.; Weinberg, C.; Korelitz, T.; Andrews, R. Three-dimensional modeling may improve surgical education and clinical practice. Surg. Innov. 2016, 23, 189–195. [Google Scholar] [CrossRef]
- Goo, H.W.; Park, S.J.; Yoo, S.-J. Advanced medical use of three-dimensional imaging in congenital heart disease: Augmented reality, mixed reality, virtual reality, and three-dimensional printing. Korean J. Radiol. 2020, 21, 133. [Google Scholar] [CrossRef]
- Ganguli, A.; Pagan-Diaz, G.J.; Grant, L.; Cvetkovic, C.; Bramlet, M.; Vozenilek, J.; Kesavadas, T.; Bashir, R. 3D printing for preoperative planning and surgical training: A review. Biomed. Microdevices 2018, 20, 1–24. [Google Scholar] [CrossRef]


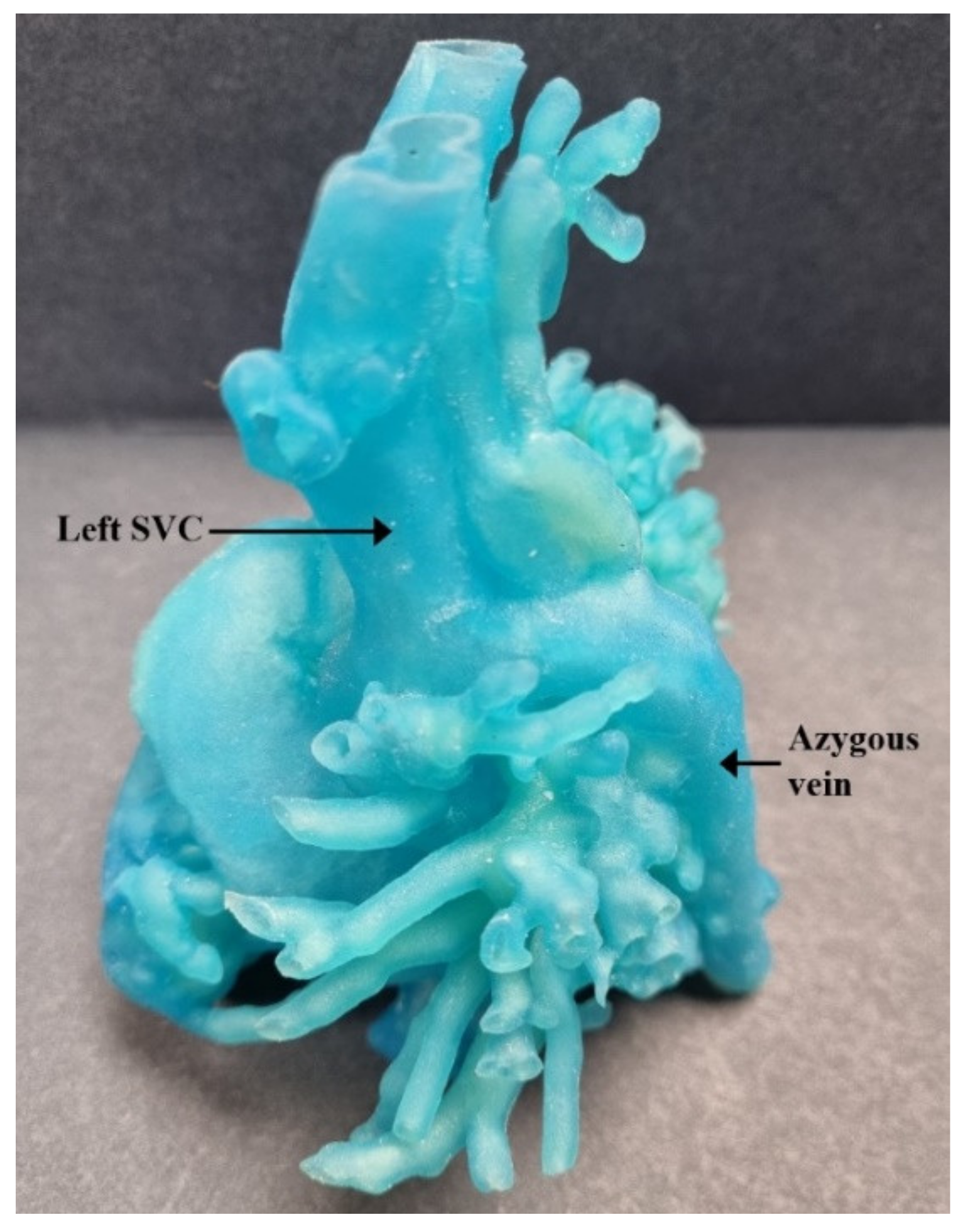
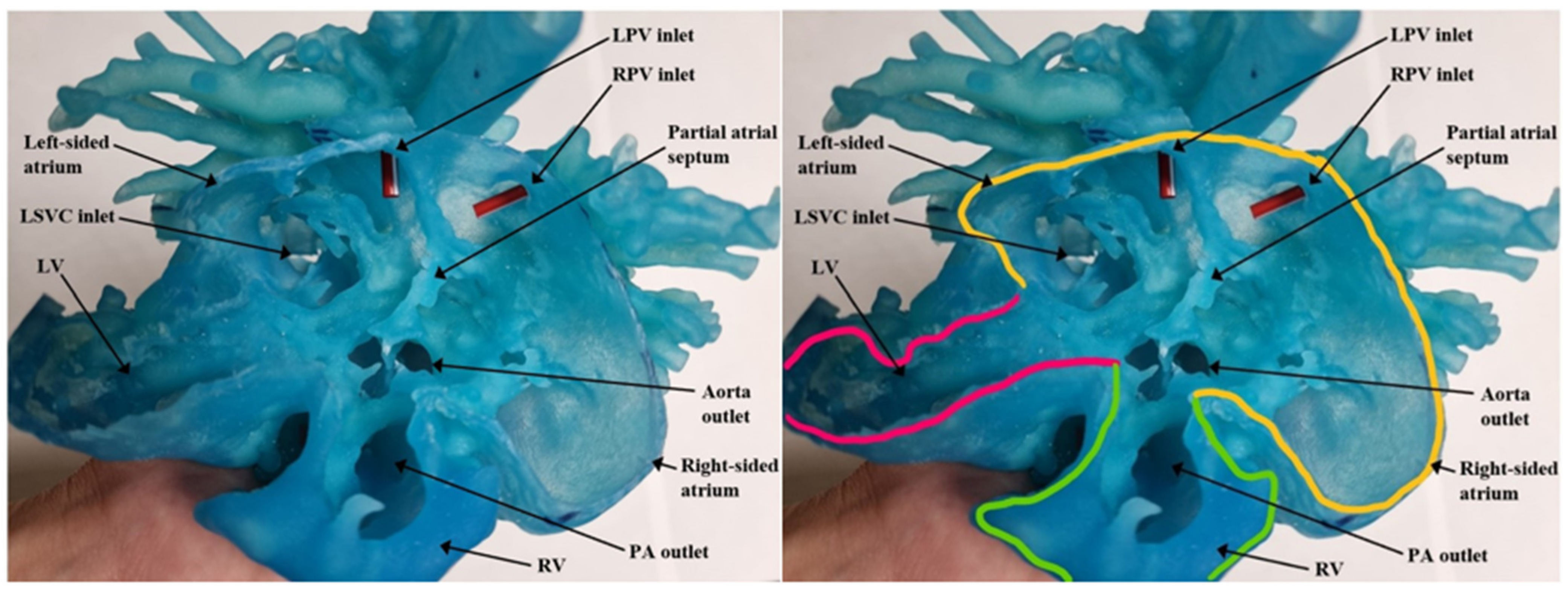


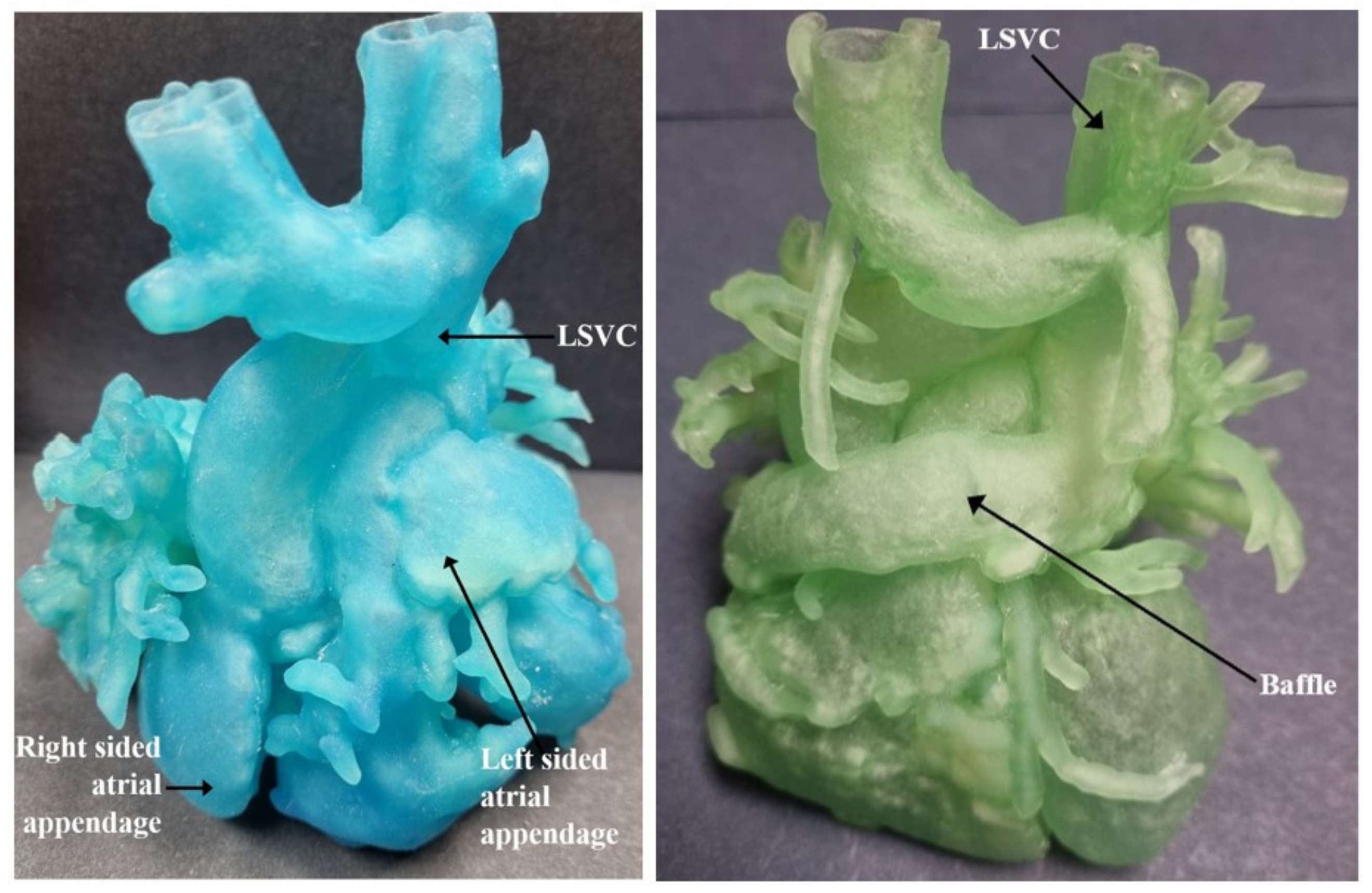
| Authors | CHD Diagnosis | Age | Prior Surgical Intervention | Impact of 3D Printed Model | Surgical Intervention Performed |
|---|---|---|---|---|---|
| Riesenkampff et al. [19] | Case 1: DORV, LVOTO, coarctation of the aorta | 20 months | PA banding, CoA repair | Defined VSD morphology and its relation to outflow tracts | Biventricular repair with creation of baffle between LV to aorta |
| Case 2: DORV, multiple VSDs, valvar pulmonary stenosis | 11 months | None | Biventricular repair with creation of unobstructed tunnel from LV to aorta | ||
| Case 3: Tetralogy of Fallot, large VSD, severe pulmonary stenosis, tricuspid valve straddling | 9 months | Infundibulectomy | Biventricular repair | ||
| Case 4: L-TGA, pulmonary atresia, VSD | 19 years | Enlargement of PA bifurcation | Biventricular repair | ||
| Valverde et al. [31] | TGA, subaortic VSD, severe pulmonary stenosis | 1.5 years | BT shunt | Defined cardiac anatomy and surgical plan with placement of intraventricular patch | Biventricular repair with Nikaidoh operation |
| Farooqi et al. [20] | DORV, non-committed VSD | 7 years | Bidirectional Glenn procedure | Defined positioning of the ventricles, VSD morphology, and great arteries | Biventricular repair with VSD closure and baffle creation to the aortic root, RV to RPA conduit placement, Glenn anastomosis takedown |
| Garekar et al. [21] | Case 1: DORV, VSD, side by side great arteries with aorta to the right, mild pulmonary stenosis | 7 months | None | Defined cardiac anatomy and helped with surgical approach | Biventricular repair with baffle from LV to aorta |
| Case 2: DORV, large VSD, D-TGA, severe pulmonary stenosis | 6 years | Initial single ventricle palliation | Defined cardiac anatomy and helped decide single ventricle vs. biventricular repair | Biventricular repair with LV to aorta baffle and RV to PA conduit | |
| Case 3: DORV, L-TGA, large VSD, severe pulmonary stenosis | 11 years | Bidirectional Glenn procedure | Defined cardiac anatomy and pre-surgical plan (Hemi-mustard procedure) | 1.5 ventricular repair with hemi-mustard procedure (Bidirectional Glenn left intact) | |
| Kappanayil et al. [22] | Case 1: Situs inversus, dextrocardia, DORV, large VSD, side by side great arteries, severe pulmonary stenosis, bilateral SVC | 18 years | None | Defined VSD morphology and its relation to outflow tracts, and challenges in routing LV to aorta | Biventricular repair with Rastelli operation |
| Case 2: Situs solitus, mesocardia, large conoventricular VSD, mid-muscular and apical VSD, pulmonary atresia, severe tricuspid regurgitation, moderate ventricular dysfunction | 12 years | Right modified BT shunt-1 month; Left modified BT shunt-2 years; Bidirectional Glenn shunt-7 years | Defined complex ventricular septum anatomy and surgical plan for intraventricular tunneling | Biventricular repair with Rastelli operation and tricuspid valve repair | |
| Case 3: Situs inversus, mesocardia, L-TGA, pulmonary atresia, large VSD, moderate RV hypoplasia | 15 years | None | Defined complex anatomy and determined inability to perform full biventricular repair | 1.5 ventricular repair with atrial switch, Rastelli operation; bidirectional Glenn shunt left intact | |
| Anwar et al. [23] | Situs inversus, dextrocardia, DORV, L-TGA, VSD | 8 months | None | Defined VSD morphology and its relation to outflow tracts and surgical approach | Biventricular repair |
| Valverde et al. [28] | Case 1: DORV, D-TGA, aortic arch hypoplasia | 1 month | Hybrid Norwood Procedure | Changed conservative management to biventricular repair | Biventricular repair with arterial switch, VSD closure, aortic arch repair, takedown of PA band |
| Case 2: Heterotaxy syndrome, dextrocardia, isomerism of left-sided atrial appendages, bilateral SVCs, interrupted IVC with azygos continuation | 34 years | Septation of common atrium with two patch technique at 2 years and 34 years | Biventricular repair with atrial septation, redirection of systemic flow to tricuspid valve and pulmonary venous flow to mitral valve | ||
| 7 patients with various diagnoses: DORV, D-TGA, VSD, pulmonary stenosis, coarctation of the aorta, interrupted aortic arch | 7 months –1 year | Various interventions: PA banding, BT shunt, atrial septostomy, PDA stenting, intraventricular baffle from LV to aorta, Yasui operation | Maintained plan for biventricular repair | Biventricular repair | |
| 4 patients with various diagnoses: DORV, AV canal defect, VSD, pulmonary stenosis, common atrium, single left SVC | 18 months –4 years | Various interventions: PA banding, Bidirectional Glenn shunt | Changed plan from staged single ventricle palliation to biventricular repair | Biventricular repair | |
| 7 patients with various diagnoses: DORV, TGA, VSD, hypoplastic aortic arch, coarctation of the aorta, pulmonary stenosis | 1 month to 11 years | Various interventions: PA banding, Bidirectional Glenn shunt, arterial switch operation, coarctation of the aorta repair, LV to PA homograft, VSD closure | Modified plan for biventricular repair | Biventricular repair | |
| Bhatla et al. [32] | DORV, large VSD, hypoplastic aortic arch | 2 months | None | Defined relationship of great arteries to VSD and conal septum | Biventricular repair with Yasui operation and aortic arch reconstruction |
| Bhatla et al. [24] | DORV, aorta rightward and posterior of PA, large oblong VSD nearly intersected with large conal septum | Newborn | None | Defined complex anatomy and surgical plan for VSD closure via TV approach | Biventricular repair with VSD closure via TV approach, LV to aorta baffle, and arterial switch |
| Hoashi et al. [25] | 11 patients with various diagnoses: DORV, L-TGA, D-TGA, interrupted aortic arch type B, tetralogy of Fallot with pulmonary atresia and MAPCAs | 1 month–5.9 years | Various interventions: PA banding, BT shunt, hybrid stage 1 palliation with PA banding and PDA stenting, RV-PA conduit with PA-plasty | Defined VSD morphology and its relation to outflow tracts and surgical approaches for inexperienced surgeon | Biventricular repair |
| Agarwal A. [27] | Heterotaxy syndrome with interrupted IVC with azygos continuation to SVC, atrial situs ambiguous, AV discordance, balanced AV canal defect, L-looped ventricles, VA concordance | 4 years | None | Defined complex cardiac anatomy and pre-surgical plan | Biventricular repair with repair of AV canal defect and atrial switch procedure (Mustard-like operation) |
| Vettukattil et al. [26] | Case 1: Hypoplastic RV, situs inversus totalis, right atrial isomerism, unbalanced AV canal defect, bilateral SVCs, TAPVC | 3 years | BT shunt, bidirectional Glenn shunt, ligation of left SVC, TAPVC repair | Defined feasibility of 1.5 ventricular repair and pre-surgical plan | 1.5 ventricular repair |
| Case 2: DORV with pulmonary atresia, situs inversus totalis, subaortic VSD with single coronary artery, bilateral SVC | 8 years | Unknown initial stage palliation, Bidirectional Glenn shunt | Assessed RV for biventricular repair, planned surgical approach for RV to PA conduit | Biventricular repair | |
| Case 3: Pulmonary atresia with intact ventricular septum | 26 years | BT shunt, bidirectional Glenn shunt | Planned right ventriculotomy and defined tricuspid valve anatomy | 1.5 ventricular repair | |
| Present case [6] | Heterotaxy syndrome with partial LPA sling, partial AV canal with a common atrium, atrial situs ambiguous, interrupted IVC, VSD, and PDA | 5 months | PDA occlusion, clipping and division of the anomalous LPA | Defined complex anatomy and surgical plan for biventricular repair | Biventricular repair |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betancourt, L.G.; Wong, S.H.; Singh, H.R.; Nento, D.; Agarwal, A. Utility of Three-Dimensional Printed Model in Biventricular Repair of Complex Congenital Cardiac Defects: Case Report and Review of Literature. Children 2022, 9, 184. https://doi.org/10.3390/children9020184
Betancourt LG, Wong SH, Singh HR, Nento D, Agarwal A. Utility of Three-Dimensional Printed Model in Biventricular Repair of Complex Congenital Cardiac Defects: Case Report and Review of Literature. Children. 2022; 9(2):184. https://doi.org/10.3390/children9020184
Chicago/Turabian StyleBetancourt, Lauren Gabriel, Si Hui Wong, Harinder R. Singh, Daniel Nento, and Arpit Agarwal. 2022. "Utility of Three-Dimensional Printed Model in Biventricular Repair of Complex Congenital Cardiac Defects: Case Report and Review of Literature" Children 9, no. 2: 184. https://doi.org/10.3390/children9020184
APA StyleBetancourt, L. G., Wong, S. H., Singh, H. R., Nento, D., & Agarwal, A. (2022). Utility of Three-Dimensional Printed Model in Biventricular Repair of Complex Congenital Cardiac Defects: Case Report and Review of Literature. Children, 9(2), 184. https://doi.org/10.3390/children9020184





