Right Pulmonary Artery Originating from Ascending Aorta (Hemitruncus Arteriosus) with VACTERL Association in a Neonate: A Case Report
Abstract
:1. Introduction
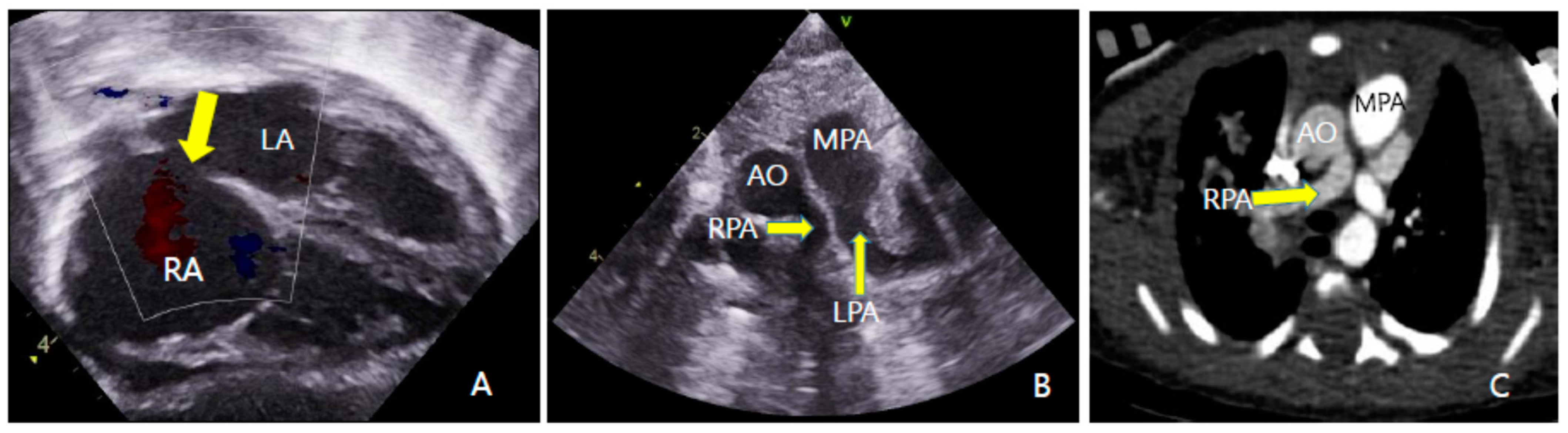
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, J.; Mao, M.; Zhang, Y.; Ai, F.-F.; Zhu, L. Congenital anal atresia with rectovestibular fistula, scoliosis, unilateral renal agenesis, and finger defect (VACTERL association) in a patient with partial bicornuate uterus and distal vaginal atresia: A case report. Medicine 2018, 9, e12822. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.D. VACTERL/VATER Association. Orphanet. J. Rare Dis. 2011, 16, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunningham, B.K.; Hadley, D.W.; Hannoush, H.; Meltzer, A.C.; Niforatos, N.; Pineda-Alvarez, D.; Sachdev, V.; Warren-Mora, N.; Solomon, B.D. Analysis of cardiac anomalies in VACTERL association. Birth Defects Res. Part A Clin. Mol. Teratol. 2013, 97, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sulaiman, R.M.; Hashmi, A.; McCrindle, B.W.; Williams, W.G.; Freedom, R.M. Anomalous origin of one pulmonary artery from the ascending aorta: 36 years’ experience from one centre. Cardiol. Young 1998, 8, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Kutsche, L.M.; Van Mierop, L. Anomalous origin of a pulmonary artery from the ascending aorta: Associated anomalies and pathogenesis. Am. J. Cardiol. 1988, 61, 850–856. [Google Scholar] [CrossRef]
- Cheng, W.; Xiao, Y.; Zhong, Q.; Wen, R. Anomalous origin of left pulmonary artery branch from the aorta with Fallot’s tetralogy. Thorac. Cardiovasc. Surg. 2008, 56, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Smith, D.W.; The VATER Association. Vertebral defects, anal atresia, T-E fistula with esophageal atresia, radial and renal dysplasia: A spectrum of associated defects. J. Pediatr. 1973, 82, 104–107. [Google Scholar] [CrossRef]
- Agochukwu, N.B.; Pineda-Alvarez, D.E.; Keaton, A.A.; Warren-Mora, N.; Raam, M.S.; Kamat, A.; Chandrasekharappa, S.C.; Solomon, B.D. Analysis of FOXF1 and the FOX gene cluster in patients with VACTERL association. Eur. J. Med. Genet. 2011, 54, 323–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubinsky, M. The VACTERL association: Mosaic mitotic aneuploidy as a cause and a model. J. Assist. Reprod. Genet. 2019, 36, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.D. The etiology of VACTERL association: Current knowledge and hypotheses. Am. J. Med. Genet. Part C Semin. Med. Genet. 2018, 178, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.D.; Pineda-Alvarez, D.E.; Raam, M.S.; Bous, S.M.; Keaton, A.A.; Velez, J.; Cummings, D.A. Analysis of component findings in 79 patients diagnosed with VACTERL association. Am. J. Med. Genet. Part A 2010, 152A, 2236–2244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Song, Y.; Cheng, T.O.; Xie, M.; Wang, X.; Yuan, L.; Yang, Y.; Wang, L. The value of transthoracic echocardiography in the diagnosis of anomalous origin of the right pulmonary artery from the ascending aorta: A single center experience from China. Int. J. Cardiol. 2015, 184, 750–754. [Google Scholar] [CrossRef]
- Dong, S.; Yan, J.; Xu, H.; Duan, Y.; Liu, C. The surgical treatment of anomalous origin of one pulmonary artery from the ascending aorta. J. Cardiothorac. Surg. 2019, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Fontana, G.P.; Spach, M.S.; Effmann, E.L.; Sabiston, D.C. Origin of the Right Pulmonary Artery from the Ascending Aorta. Ann. Surg. 1987, 206, 102–113. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, B.-S.; Kim, T.; Lee, H.D.; Ko, H.; Byun, J.-H. Right Pulmonary Artery Originating from Ascending Aorta (Hemitruncus Arteriosus) with VACTERL Association in a Neonate: A Case Report. Children 2022, 9, 194. https://doi.org/10.3390/children9020194
Shin B-S, Kim T, Lee HD, Ko H, Byun J-H. Right Pulmonary Artery Originating from Ascending Aorta (Hemitruncus Arteriosus) with VACTERL Association in a Neonate: A Case Report. Children. 2022; 9(2):194. https://doi.org/10.3390/children9020194
Chicago/Turabian StyleShin, Byeong-Su, Taehong Kim, Hyoung Doo Lee, Hoon Ko, and Joung-Hee Byun. 2022. "Right Pulmonary Artery Originating from Ascending Aorta (Hemitruncus Arteriosus) with VACTERL Association in a Neonate: A Case Report" Children 9, no. 2: 194. https://doi.org/10.3390/children9020194
APA StyleShin, B.-S., Kim, T., Lee, H. D., Ko, H., & Byun, J.-H. (2022). Right Pulmonary Artery Originating from Ascending Aorta (Hemitruncus Arteriosus) with VACTERL Association in a Neonate: A Case Report. Children, 9(2), 194. https://doi.org/10.3390/children9020194





